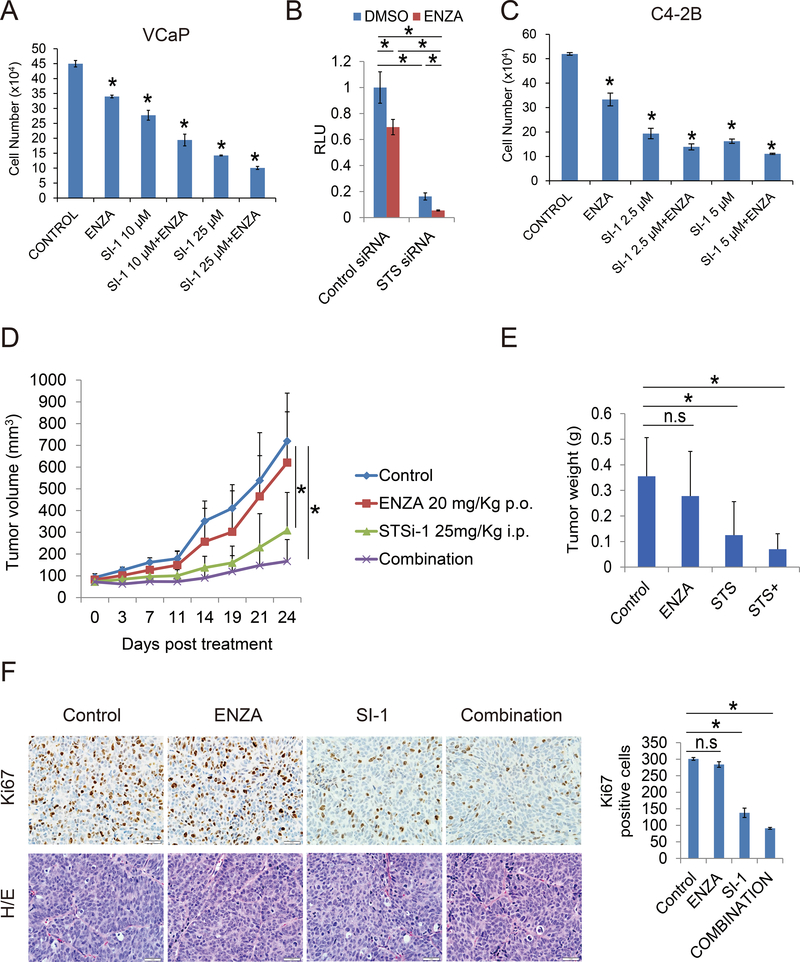
Figure 6.
STS inhibitors improve enzalutamide treatment in vitro and in vivo. A. VCaP cells were treated with 10 μM or 25 μM SI-1 or SI-2 with or without 20 μM enzalutamide for 3 days, cell number was determined. B. VCaP cells were transiently transfected with control siRNA or STS siRNA with PSA luciferase plasmid for 3 days and thentreated with 10 μM enzalutamide for 24 hours. Luciferase activity was determined. C. C4–2B cells were treated with 2.5 μM or 5 μM SI-1 or SI-2 with or without 20 μM enzalutamide for 3 days and cell number was determined. D-E. Mice bearing VCaP xenografts were castrated and the replase tumors were treated with vehicle control, enzalutamide (25 mg/Kg p.o), SI-1 (25 mg/Kg i.p), or their combination for 3 weeks, tumor volumes were measured twice weekly and the tumors were collected and weighed after 3 weeks treatment. F. IHC staining of Ki67 and H/E staining in each group was performed and quantified as described in methods. * p<0.05.