Figure 4.
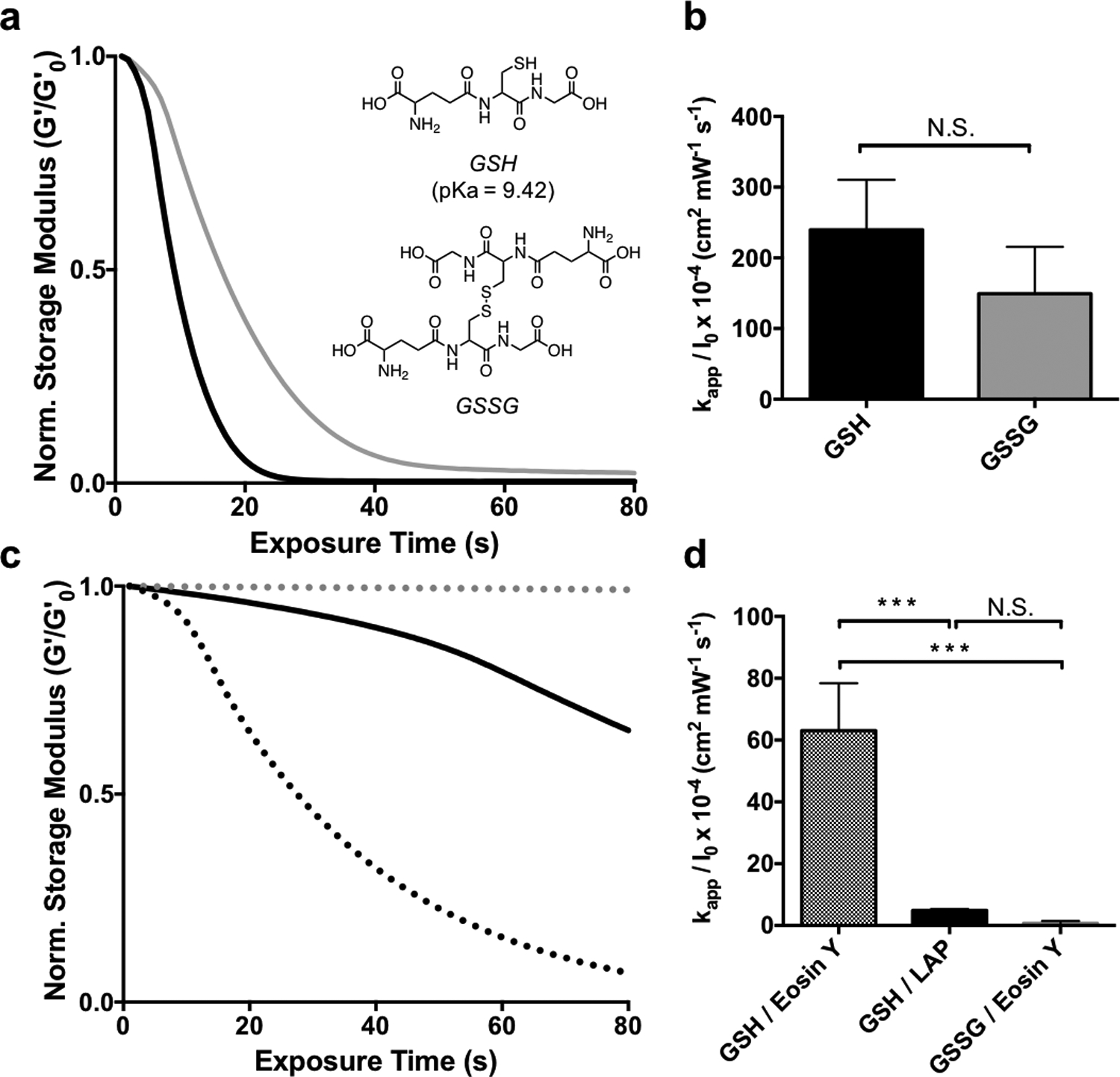
The effect of thiol oxidation state and initiator type on allyl sulfide hydrogel degradation kinetics. a AS-PA hydrogels equilibrated with glutathione (GSH, black) and glutathione disulfide (GSSG, grey) display similar degradation kinetics when degraded using 1mM LAP and 365nm light (5 mW cm−2). b Apparent rate constants for photodegradation of AS-PA using GSH and GSSG are shown. Significance determined by t test, N=3, mean ± s.d.. c Degradation is slower using GSH and LAP at higher wavelengths, such as 405 nm light (solid black). However, using 405 nm light and the photoinitiator eosin Y, degradation is more rapid with GSH (black dot), while GSSG (grey dot) exhibits little degradation. d Apparent rate constants for photodegradation of AS-PA using thiols, GSH and GSSG, and photoinitiators, LAP and eosin y, are shown. Significance determined by one way ANOVA, N=3, mean ± s.d., ***p < 0.001. Hydrogels equilibrated with 15 mM soluble thiol and 1 mM photoinitiator at pH 7.4 were exposed to light (365 nm or 405/436, 5 mW cm−2) at t = 0. All rheological plots show a representative trace from N=3.
