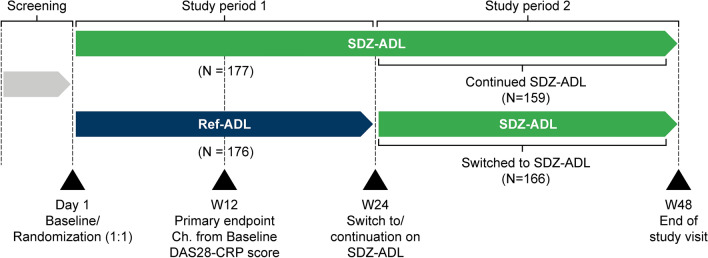
Fig. 1.
Study design resembled ref-ADL ARMADA trial [25]. Study treatment in Study Period 1: SDZ-ADL or ref-ADL 40 mg/0.8 mL sc injection from Day 1 to W22. Study treatment in Study Period 2: patients with at least moderate EULAR response switched to SDZ-ADL from W24 until W46. In Study Periods 1 and 2, last injections were administered at W22 and W46, respectively; last patient assessments were performed at W24 and W48, respectively. CRP C-reactive protein, DAS disease activity score, EULAR European League Against Rheumatism, Ref-ADL reference adalimumab, sc subcutaneous, SDZ-ADL Sandoz biosimilar adalimumab, W week