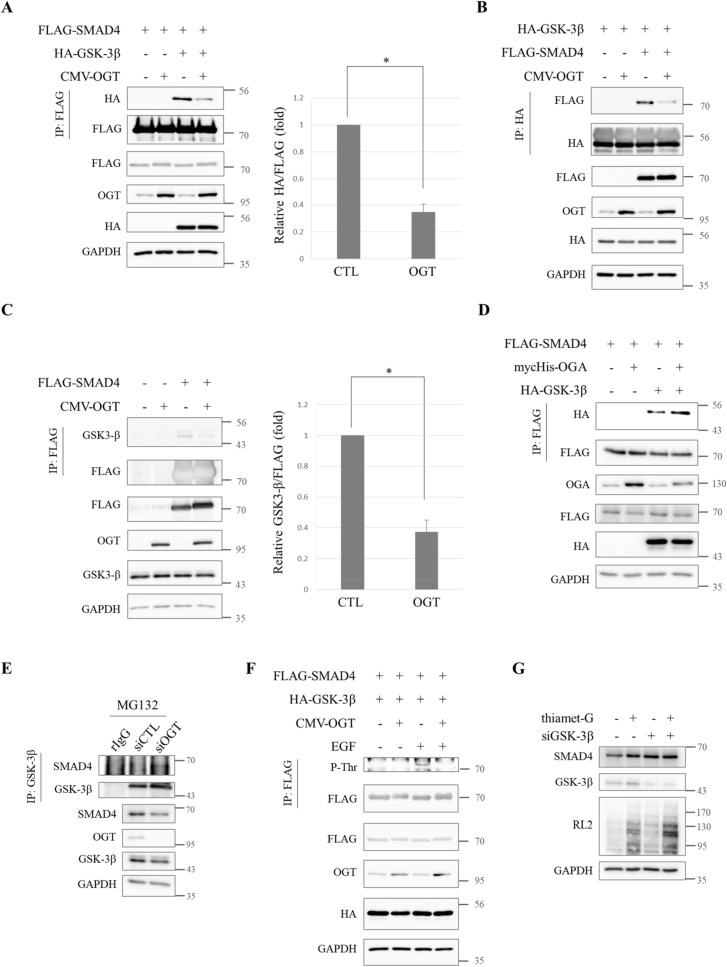
Figure 2.
The interaction between SMAD4 and GSK-3β is interrupted by O-GlcNAc. (A) Immunoblots indicating hampered interactions between HA-GSK-3β and FLAG-SMAD4 when pCMV-OGT was overexpressed. Expression vectors encoding FLAG-SMAD4, HA-GSK-3β and pCMV-OGT were co-transfected in HEK293 cells for 24 h and whole lysates were subjected to immunoprecipitation using anti-FLAG. The precipitates were then immunoblotted with anti-HA to show the interactions between FLAG-SMAD4 and HA-GSK3β. Data are given as mean ± standard error (n = 3). The significance was *p < 0.05. P values were calculated by Student’s t test. Full-length blots are presented in Supplementary Fig. S4. (B) Western blot analyses showing alleviation of co-immunoprecipitated FLAG-SMAD4 in anti-HA-GSK-3β precipitates under pCMV-OGT-overexpressing conditions. HEK293 cells were transfected with plasmids encoding FLAG-SMAD4, HA-GSK-3β, and pCMV-OGT for 24 h and whole lysates were subjected to co-immunoprecipitation. Full-length blots are presented in Supplementary Fig. S4. (C) Immunoblots showing decreased endogenous GSK-3β interactions in anti-FLAG-SMAD4 immunoprecipitates. Constructs encoding FLAG-SMAD4 and pCMV-OGT were co-transfected into HEK293 cells for 24 h. An immunoprecipitation assay was then performed with anti-FLAG. The associated endogenous GSK-3β was detected by immunoblots using anti-GSK-3β. Data are given as mean ± standard error (n = 3). The significance was *p < 0.05. P values were calculated by Student’s t test. Full-length blots are presented in Supplementary Fig. S4. (D) Western blot analyses indicating increased interactions between FLAG-SMAD4 and HA-GSK3β when myc-His-OGA was overexpressed. HEK293 cells were transfected with plasmids encoding FLAG-SMAD4, HA-GSK-3β, and myc-His-OGA for 24 h and total lysates were subjected to co-immunoprecipitation using anti-FLAG. The precipitates were then immunoblotted with anti-HA to observe the interactions between FLAG-SMAD4 and HA-GSK-3β. Full-length blots are presented in Supplementary Fig. S4. (E) Immunoblots showing increased interactions between endogenous SMAD4 and GSK-3β when OGT was knocked down. A549 cells were treated with small interfering RNA-targeting OGT for 48 h and treated with 20 µM MG132 6 h prior to being harvested. An immunoprecipitation assay was then performed with anti-GSK-3β. The associated endogenous SMAD4 was detected by immunoblots using anti-SMAD4. Full-length blots are presented in Supplementary Fig. S4. (F) Immunoblot analyses showing reduced phospho-threonine levels of FLAG-SMAD4 in the presence of pCMV-OGT overexpression. HEK293 cells were transfected with plasmids encoding FLAG-SMAD4, HA-GSK-3β, and pCMV-OGT for 48 h. Cells were pre-treated with 100 ng/ml EGF for 24 h to enhance signaling. Full-length blots are presented in Supplementary Fig. S4. (G) Western blot data demonstrating no SMAD4 accumulation in GSK-3β k/d A549 cells. Cells were treated with small interfering RNA-targeting GSK-3β for 60 h and supplemented with 10 μM thiamet-G for 48 h. Full-length blots are presented in Supplementary Fig. S4.