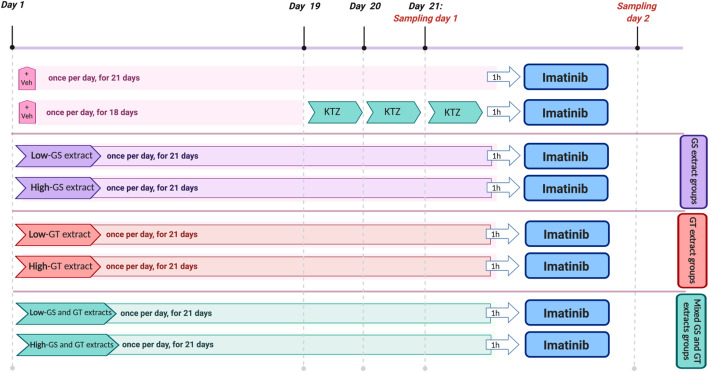
Fig. 1.
Scheme of the pharmacokinetics study design in different groups: control (imatinib only), positive control (+KTZ), grape seed extract groups (“+low-GS” and “+high-GS”), green tea extract groups (“+low-GT” and “+high-GT”, and mixed extracts groups (“+low-GS and GT” and “+high-GS and GT”). Imatinib in all groups was given as a single dose of imatinib (30 mg/kg; dissolved in DMSO) by intragastric (IG) oral gavage. KTZ was given as a single daily dose (75 mg/kg; IG, dissolved in DMSO), for three consecutive days. Low dose refers to 50 mg/kg/day, IG, of GS and/or GT extracts. High dose refers to 100 mg/kg/day, IG, of GS and/or GT extracts. Extracts were administered for 21 days before imatinib administration. Control and positive control groups were given an equivalent volume of DMSO (extracts’ vehicle, IG; “+Veh”) for 21 and 18 days, respectively