Figure 1.
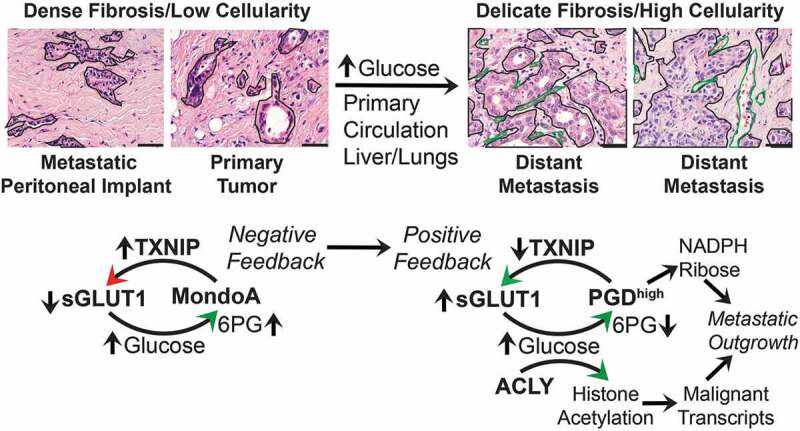
Pancreatic cancers suppress negative feedback of glucose transport to activate metaboloepigenetic programs that support distant metastasis. Left panels: hematoxylin and eosin (H&E) images show that primary pancreatic ductal adenocarcinomas (PDACs) and metastatic peritoneal implants are densely fibrotic with relatively low tumor cellularity. The invasive tumor glands (outlined) are embedded within a dense (scar-like) and hypovascular stroma. Genetic drivers are selected early in this microenvironment to activate scavenging pathways, such as autophagy and macropinocytosis, that promote tumor growth under these hypoglycemic conditions. In this context, MLX interacting protein (MLXIP, better known as MondoA) can appropriately sense glucose uptake if it occurs (possibly indirectly through 6-phosphogluconate (6PG) or other sugar metabolites) and prevent excessive consumption by activating expression of thioredoxin-interacting protein (TXNIP) for endocytosis of glucose transporter protein type 1 (GLUT1) off the cell surface (sGLUT1). Right panels: A subset of PDAC subclones are exposed to microenvironments that are more nutrient/glucose replete. This could occur within unusually well-vascularized regions of primary tumor, within the circulation, or at the metastatic site(s) itself (liver, lungs). This is depicted by typical H&E images from PDAC liver metastases, showing metastatic glands with higher tumor cellularity (outlined in black) growing in a more delicate (thin) fibrotic stroma with a rich microvascular network (green outlines). Such microenvironments may allow selection and clonal expansion of PDAC cells that acquire activation of phosphogluconate dehydrogenase (PGD), which suppresses the MondoA-TXNIP negative feedback loop. Mechanistically, high PGD catalysis (PGDhigh) (over)-consumes glucose-derived 6PG, which prevents MondoA-mediated activation of TXNIP. Suppression of TXNIP allows sGLUT1 to remain at the cell surface with corresponding increases in glucose import. The glucose fuels PGDhigh catalysis by replenishing depleted 6PG substrates. In parallel, glucose-derived citrate is provided to ATP citrate lyase (ACLY) for production of the bulk acetyl groups required to hyperacetylate histones within active chromatin. It is further possible that the PGD protein is itself acetylated into a more (hyper)-active conformation that helps facilitate rapid consumption of 6PG substrates. PGDhigh synthesizes ribulose/ribose (for nucleotides) and nicotinamide adenine dinucleotide phosphate (NADPH, for lipids and redox balance), while global histone hyperacetylation is permissive for full transcriptional activation of malignant gene transcripts. These two glucose-fueled tumorigenic activities synergize to support or even accelerate widespread metastatic outgrowth. H&E scale bars: 50 µm
