ABSTRACT
Dopamine (3-hydroxytyramine or 3,4-dihydroxyphenethylamine) has many functions in animals, but also shows several other functions in plants. Since the discovery of dopamine in plants in 1968, many studies have provided insight into physiological and biochemical functions, and stress responses of this molecule. In this review, we describe the biosynthesis of dopamine, as well as its role in plant growth and development. In addition, endogenous or exogenously applied dopamine improved the tolerance against several abiotic stresses, such as drought, salt, and nutrient stress. There are also several studies that dopamine contributes to the plant immune response against plant disease. Dopamine affects the expression of many abiotic stresses related genes, which highlights its role as a multi-regulatory molecule and can coordinate many aspects of plant development. Our review emphasized the effects of dopamine against environmental stresses along with future research directions, which will help improve the yield of eco-friendly crops and ensure food security.
KEYWORDS: 3,4- dihydroxy; phenethylamine; plant; biosynthetic pathway; abiotic stress; biotic stress
1. Introduction
Dopamine, along with norepinephrine and epinephrine, is a type of catecholamine found throughout the plant and animal kingdoms. Catecholamines are characterized as biogenic amines possessing a 3,4-dihydroxy-substituted phenyl ring, and are widespread in animals where they are well-known neurotransmitters.1 Dopamine is a nitrogen-containing organic compound with the molecular formula of C8H11NO2, its molecular weight is 153.18 (Figure 1a). It is sensitive to light and easy to oxidize in the presence of oxygen. In humans, dopamine serves a wide range of well-defined functions, including processes involved in reward, addiction, control of coordinated movement, metabolism, and hormonal secretion.2 Correspondingly, the dysregulation of the dopaminergic system has been implicated in diseases such as schizophrenia; Parkinson’s disease; depression; attention deficit hyperactive disorder; nausea and vomiting; and more recently, the autism spectrum disorder.3,4
Figure 1.
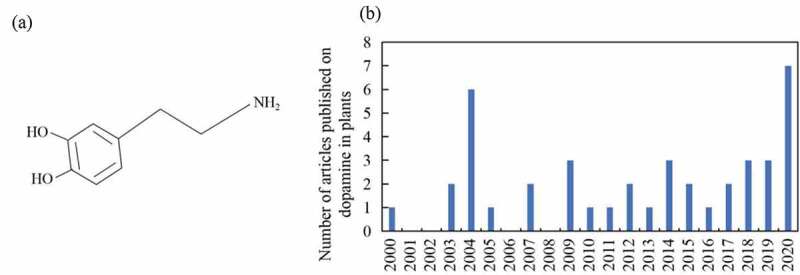
(a) Structures of dopamine and (b) evolution of the number of articles related with dopamine in plants from 2000 to 2020 (Jan to Sep)
Due to population growth and climate change, the negative effects of environmental stress on plant production increased in many regions of the world.5 The abiotic stresses such as drought, salinity, and nutrient deficiencies severely affect the growth, development, and metabolism of plants.6,7 Recently, many approaches have been used to overcome abiotic stresses in plants.8 Chemical priming is a promising field in crop stress physiology. The use of compounds as initiators has been found to significantly improve plant tolerance to a variety of biological and abiotic stresses.9
Dopamine can promote the growth of plants under various stressful environments.10–14 More recent studies have shown that dopamine can enhance tolerance to drought, salt stress, and nutrient deficiency in plants.11–13 In addition, dopamine can improve the ability of plants to resist biological stressors. However, the number of articles on dopamine in plants is still small. The number of papers was less than 10 each year (Figure 1b). In order to better promote the development of this field, this paper reviewed the effects of dopamine on abiotic stress in plants, and provided the future research direction for the utilization of dopamine for sustainable production of crops.
2. Endogenous dopamine present in different plants
Considering the multiple function of dopamine in animals, investigation was carried out on the plants, and dopamine was detected in 1968.1,15 Dopamine content varies considerably among species, from a few nanograms to several micrograms per gram. For example, dopamine is found at high concentration in the pulp of yellow banana (Musa acuminata), red banana (Musa sapientum var. baracoa), the spathes of Araceae inflorescences, fuerte avocado (Persea americana), and plantain (Plantago major).1 However, the dopamine content in oranges, tomatoes, apples and other plants being relatively low, with a fresh weight of less than 1 µg per gram.16,17 These notable variations of endogenous dopamine content among different plant species suggested that dopamine function varied from plant to plant. The content of dopamine in the number of plants is presented in Table 1.
Table 1.
Dopamine content in different parts of plants
| Species | Detected Plant Parts | Dopamine content |
|---|---|---|
| Yellow banana (Musa acuminata) | Fruit pulp | 42 μg/g FW |
| Red banana (Musa sapientum var. baracoa) | Fruit pulp | 55 μg/g FW |
| Plantain (Plantago major) | Fruit pulp | 5.5 μg/g FW |
| Fuerte avocado (Persea americana) | Fruit pulp | 4 μg/g FW |
| Cavendish banana | Fruit pulp | 2.5–10 μg/g FW |
| Cavendish banana | Fruit peel | 100 μg/g FW |
| Potato (Solanum tuberosum var. Desiree) | Leaves | 2–7 μg/g FW |
| Potato (Solanum tuberosum var. Desiree) | Tubers | < 0.5 μg/g FW |
| Portulaca (Portulaca oleracea L.) | 39 μg/g DW | |
| Ryegrass (Lolium perenne L.) | Seeds | 37.66 μg/g FW |
| Cocoa (Theobroma cacao) | Been powder | 1 μg/g FW |
| Broccoli (Brassica olereacea var. italica) | 1 μg/g FW | |
| Brousel sprouts (Brassica olereacea var. gemmifera) | 1 μg/g FW | |
| Oranges (Citrus sinensis) | < 1 μg/g FW | |
| Tomatos (Lycopersicon esculentum) | < 1 μg/g FW | |
| Aubergine (Solanum melanogena) | < 1 μg/g FW | |
| Spinach (Spinacia oleracea) | < 1 μg/g FW | |
| Beans (Phaseolus vulgaris) | < 1 μg/g FW | |
| Peas (Pisum sativum) | < 1 μg/g FW | |
| Apples (Malus domestica Borkh.) | ROOTS | 5–6 ng/g FW |
| Apples (Malus domestica Borkh.) | Leaves | < 10 ng/g FW |
3. Dopamine biosynthetic pathway
The biosynthetic pathways of catecholamines in plants (Figure 2) are similar to those in mammals. There are two pathways, and the precursor of both is tyrosine.18,19 The first pathway starts with the decarboxylation of tyrosine by tyrosine decarboxylase (TYDC) to produce tyramine, which is then hydroxylated by monophenol hydroxylase (MH) to generate dopamine. The second pathway begins with the hydroxylation of tyrosine by tyrosine hydroxylase (TH) to produce levodopa (L-dopa), which is then decarboxylated by dopa decarboxylase (DD) to produce dopamine.18,20 However, there is variability between the biosynthetic pathways for dopamine in different plants.
Figure 2.
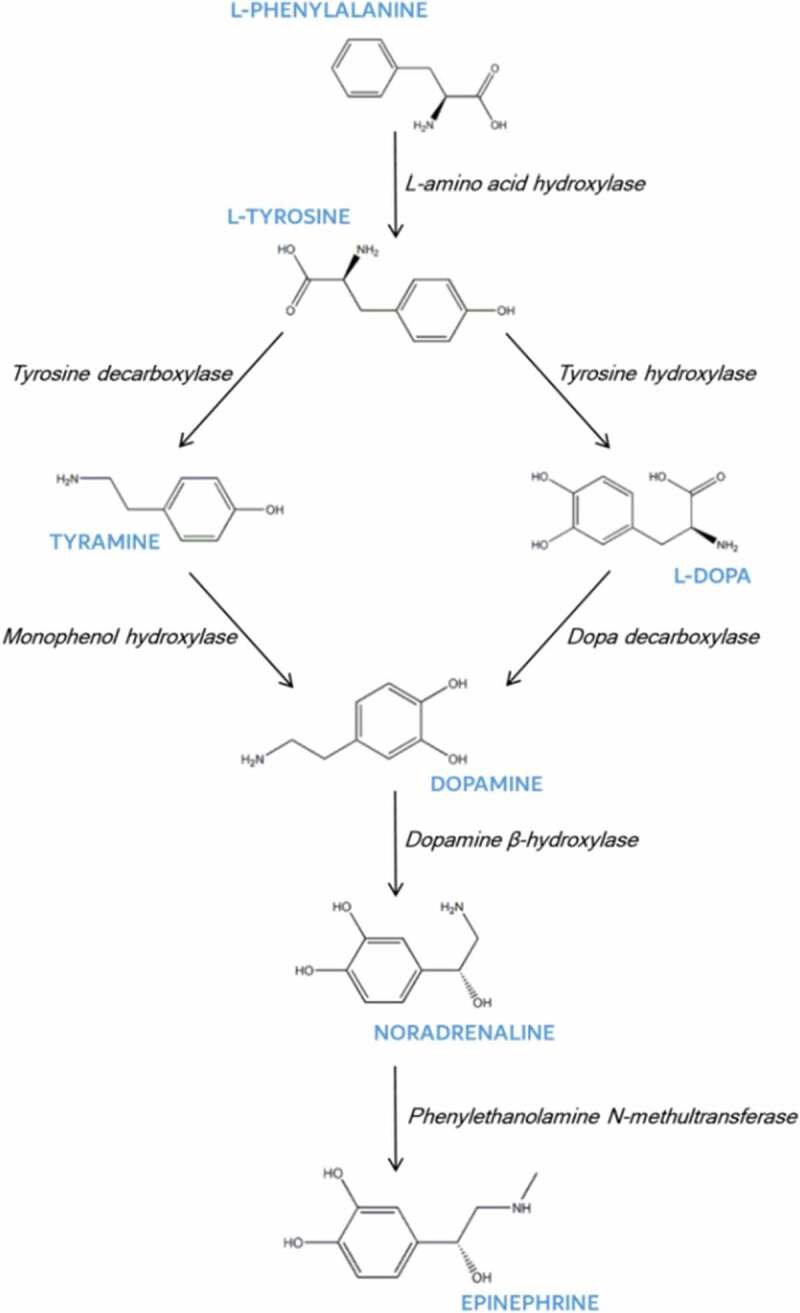
Dopamine biosynthetic pathways in plants
Indeed, the biosynthesis and catabolism of catecholamines have been described in several systems where differences can easily be identified. For example, tyrosine in Musa sapientum is first hydroxylated to form L-dopa, and then decarboxylated to form dopamine, while the synthesis of dopamine in cacti (Opuntia stricta) and purslane (Portulaca oleracea L.) initiated with the decarboxylation of tyrosine.21 Furthermore, in the peyote cactus Lophophora williamsii, phenylalanine is hydroxylated to tyrosine, which is further hydroxylated to L-dopa or decarboxylated to tyramine.22
In addition to variability in catecholamines biosynthetic pathways in plants, their synthesis is also influenced by environmental factors.11 For example, the treatment of potato plants with abscisic acid (ABA) can increase the activity of TYDC, DD, and TH; high salt treatment increased the activity of TYDC; ultraviolet exposure increased the activity of DD; drought increased the activity of TH and DD; low-temperatures can reduce the activity of DD; and dark treatment and red light treatment can inhibit the activity of TYDC, TH, and DD.11
4. Plant growth and development
Some studies have suggested that catecholamines can interact with plant hormones. For example, treatment with ABA significantly increases the level of dopamine in potato plants.23 The hormone GA3 induced hypocotyl elongation in lettuce seedlings,24 and dopamine stimulated GA3 action in isolated lettuce hypocotyls.25 Another study demonstrated that exogenous dopamine (5–100 μL) stimulated ethylene biosynthesis in illuminated chloroplast lamellae from sugar beet leaves.26 In that study, dopamine was shown to function as a cofactor in reducing monovalent oxygen, which is necessary for the formation of ethylene. It is known that auxins promote the growth of stems and coleoptile while inhibiting the growth of roots.27 The study by Kulma and Szopa showed that dopamine is key to the growth of lettuce hypocotyls,1 which fits with the observation that dopamine inhibited oxidation of the auxin IAA by reducing the expression of IAA oxidase genes.28 Catecholamines like dopamine are dihydroxyphenols, and biosynthetic dihydroxyphenols have been shown to inhibit IAA oxidase.29,30 Thus, by inhibiting IAA oxidase in the roots, dopamine increases the auxin content, high levels of which can inhibit root growth. Therefore, this could be the mode of action by which dopamine inhibits root growth, as was observed in the growth of soybean seedling roots.31
Carbohydrate levels changed in plants that transformed with dopamine synthesis genes showed that dopamine are linked with sugar metabolism.23 In previous study, overexpression of TYDC in potatoes increased the content of glucose and sucrose.32 Increased expression of human dopamine receptor in potatoes resulted in increased sucrose, glucose, and fructose contents.33 Apple plants pretreated with exogenous dopamine showed higher sucrose and malic acid contents but lower starch accumulation.9 Gao et al. (2020) showed that exogenous dopamine increased the content of glucose and fructose by increasing the expression of sucrose phosphate synthase (MdSPS1;6), cell wall invertase (MdCWINV1;2) and neutral invertase (MdCINV1;2) in mycorrhizal plant under salt stress.17
In addition, the cyclic adenosine monophosphate (cAMP) signaling pathway, which participates in the regulation of numerous metabolic processes in the cell, can be regulated by catecholamines like dopamine. Through this interaction, dopamine has been associated with processes including nitrogen fixation, flowering, and the photophosphorylation of chloroplasts.34,35 Protacio et al. (1992) showed that catecholamines stimulated growth in root cultures of Acmella oppositifolia and Nicotiana tabacum.36 However, as noted previously, dopamine inhibited growth in soybean roots,31 which may indicate that dopamine’s promotion of plant growth is determined by plant-specific interactions with growth hormones.37
5. Abiotic and biotic stressors
5.1. Drought stress
The stress caused by drought is one of the most common abiotic stresses and has the greatest impact on crop yields.38 Under drought conditions, plants usually close their stomata to minimize water loss, at the cost of reduced photosynthetic capacity.11,39 In addition, drought directly affects the absorption of nutrients by plants,40 which reduces growth rates and ultimately leads to a reduction in the accumulation of biomass.41 A plant’s water status can be observed by looking at indicators like relative water content (RWC), leaf water potential, osmotic potential, pressure potential, and transpiration rate,42 all of which are significantly affected by drought. Drought stress can also lead to the production and accumulation of ROS (e.g.,2− O2,1H2O2, RO, and OH−) in plants, which can have harmful effects.11 The expression of the TYDC gene was induced in both Arabidopsis and Malus hupehensis by drought stress.12,43 However, the effects of drought can be alleviated, the overexpression of TH can significantly increase the absorption and utilization of nutrients by plants, thereby improving their drought resistance.43
Dopamine can also reduce the impact of drought conditions by increasing the photosynthetic rates of plants. Dopamine was observed to increase the net photosynthetic rate in apple seedlings during drought conditions.9 Furthermore, under drought conditions, plants pretreated with dopamine had higher intrinsic water-use efficiencies (WUE) than those that were not.9 WUE is an important indicator of a plant’s acclimation status to drought conditions and can determine its tolerance to drought.44 Decreases in photosynthetic rate under drought conditions are related to disturbances to the photosynthetic pigments in leaves.45 Exogenous dopamine significantly suppressed the upregulation of chlorophyll degradation gene (PAO) and senescence-associate gene (SAG12) under drought stress.13 Studies have shown that dopamine increased Car, Chl a and Chl t content, keeping plants greener and reducing damage caused by drought.9 Under drought, exogenous dopamine treatment can significantly improve the water retention capacity of apple leaves, reduce leaf wilt, reduce electrolyte extravasation and adjust stomatal opening.9,11 It has been suggested that dopamine significantly increases the aperture size of the stomata under drought conditions.11 Thus, by preventing the degradation of chlorophyll and adjusting stomata, dopamine is able to alleviate the negative effects of drought on photosynthetic capacity and reduce the impact of drought stress on plant growth.11
Dopamine can also improve the antioxidant capacity of plants. Studies have shown that dopamine can significantly limit increases of H2O2 in plant leaves caused by drought stress.9 This may be related to increases in certain antioxidant enzymes like superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) in the ascorbic acid-glutathione (ASA-GSH) circulation system of the leaves under drought conditions. The up-regulated expression of certain antioxidant genes like ascorbate peroxidase (cAPX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (cGR) may also contribute to the antioxidant capacity.46,47 The strong antioxidant effect of dopamine is believed to be mainly due to: (a) Dopamine’s direct antioxidant capability. Studies have shown that dopamine’s antioxidant capacity is equal to ascorbic acid’s and catechin’s, which have strong antioxidant capacities.48 (b) Melanin, the oxidation product of dopamine, is a strong active oxygen scavenger.49 (c) Exogenous dopamine treatment can activate the antioxidant system of plants, thereby indirectly removing reactive oxygen species.9
5.2. Salt stress
Under salt stress, plants usually close or constrict their stomata to prevent water loss.50 However, closing stomata also restricts entry of CO2 to the leaf cells, thereby inhibiting photosynthesis.51 These methods of reducing the impact of salt stress can also disrupt the ion homeostasis within plants.12 The restricted ion exchange can lead to the formation of superoxide, hydrogen peroxide, hydroxyl radicals, singlet oxygen, and other ROS.52 When subjected to salt stress, the activities of the antioxidant enzymes CAT, APX, POD, and SOD in plants increase, and the degree of the increase is related to the salt tolerance of the plant.52 Furthermore, from observed increases in activity of tyrosine hydroxylase in potato tubers under salt stress, we know that salt stress can induce dopamine synthesis.32
As discussed, exogenous dopamine treatment alleviated chlorophyll degradation and improved photosynthesis under salt stress.12,53 Dopamine can reduce the content of Chl b, and in so doing increase the Chl a/b ratio and prevent the accumulation of excess of electrons, which is an adaptive mechanism in the photosynthetic electron transport chain.54 Studies have shown that exogenous dopamine can increase the degree to which stomata are opened, increasing the length and width of stomata in plants under salt stress. With dopamine treatment, the maximum stomatal openings of Malus were under 100 μM.14 Studies have shown that sugar and ABA can regulate stomatal behavior under different environmental scenarios and other studies have shown that dopamine can regulate sugar metabolism and ABA content. This may be the pathway by which dopamine is able to regulate plant stomatal behavior under salt stress.1 Dopamine has also been shown to regulate the expression of the rice aquaporin gene OsPIP1-3 under salt stress.55 It has been observed that treatment of cucumber seedlings with dopamine prior to the induction of nitrate stress inhibited the negative effects on plants by increasing the carbon metabolism, nitrogen metabolism-related enzymes and the expression of related genes.53
Under salt stress, Na+ and K+ concentrations in leaves can be important indicators of plant salt tolerance. The application of exogenous dopamine can significantly inhibit the absorption of Na+ by plants while maintaining high levels of K+ content.12 Studies have also found that exogenous dopamine can improve the water-use efficiency of plants under salt stress.12 Higher WUE can reduce the plant’s intake of salt and prevent a water deficit.56 It is believed that plant cells under salt stress can reduce the concentration of Na+ in the cells by expelling them or compartmentalizing them into vacuoles, thereby lessening the impact of the salty conditions.57 Studies have also shown that the application of exogenous dopamine can increase the expression of Na+/H+ antiporter genes (MdHKT1, MdNHX1, MdSOS1, MdSOS2, and MdSOS3) in the roots and leaves of apple plants under salt stress, thereby maintaining a higher K+/Na+ ratio within plants and alleviating the damage caused by salt stress.12
The application of dopamine can improve the antioxidant capacity of plants under salt stress by increasing the activities of SOD, POD, CAT, and APX and inhibiting the production of H2O2.12,58,59 The ASA-GSH cycle plays an important role in the salt tolerance of plants. Studies have found that the application of exogenous dopamine can significantly enhance the activities of plant dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR) under salt stress. It has also been shown that dopamine can regulate the photosynthetic oxygen reduction process.48,52 The protective effect of dopamine on plants under salt stress may be attributed to its ability to act as a natural medium for chemical analogs and to act as an oxygen reduction factor, enabling oxygen reduction to participate in energy conversion during photosynthesis.48 Therefore, dopamine can exert an important protective effect on plants under salt stress by preventing oxidative stress-induced tissue damage at the cellular level.60,61
5.3. Nutrient stress
Nutrient deficiencies can significantly reduce photosynthetic rates in plants as well as reduce the concentrations of photosynthetic pigments.11,12 Nutrient stress reduces the photosynthesis rate of plants because, when nutrients are deficient, the synthesis of biological components required for photosynthesis can be halted, which can reduce the efficiency of photosynthesis or disrupt it altogether.62 The root system is the first organ to be affected by changes in the nutrient content of the soil, so the growth status and configuration of the root system are important indicators of a plants ability to obtain nutrients.63,64 Under nutrient-deficient conditions, plant roots will adapt by continuously adjusting their physiological and structural characteristics, and the degree to which they can adapt depends on their ability to change the root architecture.65 The effects of nutrient deficiencies on plant roots manifest mainly in reductions in root length, diameter, volume, surface area, quantity, and number of root hairs.11,66 Throughout the life cycle of a plant, the realization of optimal physiological function requires a stable supply of nutrients, with a nutrient deficiency the normal growth of plants will be affected and plant biomass will be reduced.67 Furthermore, under nutrient stress the ASA-GSH circulatory system, which is an important pathway for ROS removal, can be altered, potentially reducing ROS removal efficiency.11,12
Dopamine alleviated the inhibitory effect of nutrient stress on plant photosynthesis, probably by regulating certain physiological and biochemical processes related to photosynthesis.12 It has been shown that dopamine can be used as an analog of naturally occurring substances that regulate the process of oxygen reduction in spinach photosynthesis.1 In addition, dopamine can alleviate the inhibitory effects of nutrient stress on photosynthetic rates by adjusting leaf stomatal conductance to improve the utilization of CO2 and by maintaining high concentrations of chlorophyll.12 Similarly, dopamine can alleviate the inhibitory effect of nutrient deficiency on the absorption and accumulation of large and trace elements.12 The root structure of plants is determined according to nutrient availability to best maximize absorption and utilization.63 The effect of dopamine on element absorption when nutrients are lacking is related to its ability to alter the root configuration. For example, under potassium-deficient conditions, apples were able to reconfigure their roots, like changing the length and diameter of their roots, to enhance potassium absorption and utilization.12 In addition to influencing root structure, exogenous dopamine treatment can enhance the transfer of nutrients from the roots to stems and leaves, and increase nutrient accumulation in roots. Therefore, dopamine can enhance the adaptability of plants to nutrient stress by regulating the absorption of nutrients by plants and their transfer and distribution within plants. Studies also have shown that exogenous dopamine can scavenge ROS by up-regulating the expression of ASA-GSH cycle-related genes, thereby improving resistance to nutrient stress.11
5.4. Plant diseases
Numerous studies have shown that the biosynthesis of hydroxycinnamic acid amides from tyramine, and their subsequent polymerization in the cell wall by oxidative enzymes, are an integral component of a plant’s response to a pathogen challenge.68,69 These amides, together with other cell wall-bound phenolics, are believed to create a barrier against pathogens by reducing the digestibility of the cell wall.70 Several reports have suggested that TYDC is involved in the biosynthesis of numerous secondary metabolites and thus also contributes to the plant immune response against infection.68,71 Notably, tyramine, the product of dopamine, can effectively restrict sexual reproduction and inhibit the growth of fungal hyphae.72 Therefore, it has been suggested that dopamine plays a role in disease resistance. Indeed, the expression of the TYDC gene has been shown to be higher in disease-resistant plants than in susceptible plants.73 In one case, the TYDC gene from parsley was introduced into potatoes to catalyze the metabolism of tyrosine, this increased the tyramine content in the cell wall and effectively improved the disease resistance of the potatoes.74 Similarly, introducing the poppy TYDC gene into rapeseed significantly improved the binding of tyramine to the cell wall and reduced the digestibility of cells.70 Studies have shown that TYDC expression can be induced by pathogenic bacteria as well as by methyl jasmonate,75 and that it participates in the biosynthesis of hydroxyphenylacrylamide. As a component of the cell wall, amides are considered a physical barrier against pathogens, so an increase in cell-wall amides can contribute to an increase in disease resistance.70,71
6. Conclusions and future prospects
Dopamine is a type of catecholamine which emerged as a multifunctional ubiquitous signaling molecule. In this review, we discussed the dopamine biosynthesis pathway and summarized the most relevant aspects concerning abiotic and biotic stressors (Figure 3). The expression of dopamine biosynthesis genes can be induced by drought, salt, and diseases, which may lead to an increase of endogenous dopamine content in plant (Figure 3a). Dopamine involved in plant growth and development, and against several abiotic stresses by affecting stress-related genes expression, such as chlorophyll degradation, senescence, nitrate transport, IAA oxidase, aquaporin, and carbohydrate related genes (Figure 3c; Table 2). Endogenous or exogenously applied dopamine alleviated the damage to plants caused by many abiotic and biotic stresses (Figure 3b).
Figure 3.
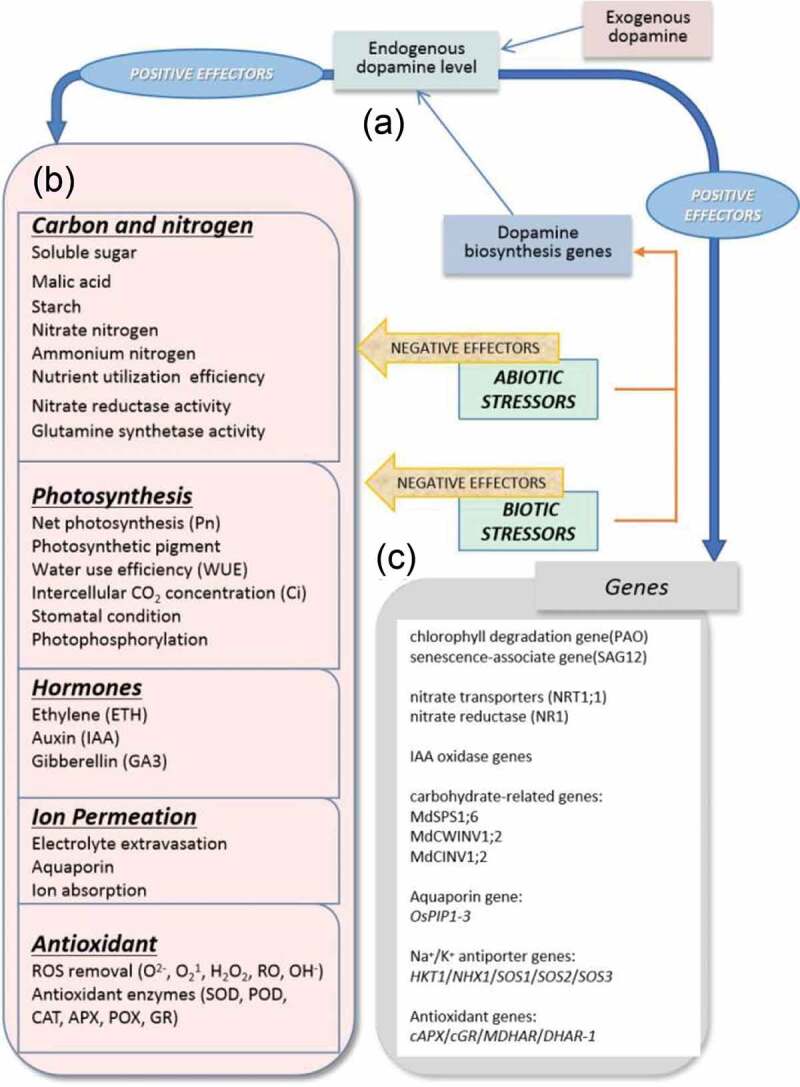
Schematic of dopamine’s positive effects on physiological processes. Abiotic and biotic stressors provoke an increase into endogenous dopamine level through the upregulation of dopamine biosynthetic genes (panel A). Stressors act as negative effectors in many physiological processes such as carbon and nitrogen metabolism, photosynthesis, and hormone levels (panel B). Endogenous dopamine can regulate the expression of many genes and regulatory factors, which can reduce the negative effects of biotic/abiotic stressors on physiological processes (panels C)
Table 2.
Dopamine biosynthesis genes and related genes regulated by dopamine in plants
| Gene names | |
|---|---|
| Dopamine biosynthetic genes | L-amino acid hydroxylase |
| Tyrosine decarboxylase | |
| Tyrosine hydroxylase | |
| Monophenol hydroxylase | |
| Dopa decarboxylase | |
| Dopamine metabolism genes | Dopamine β-hydroxylase |
| Phenylethanolamine N-methultransferase | |
| Leaf Senescence | Chlorophyll degradation gene (PAO) |
| Senescence-associate gene (SAG12) | |
| Carbohydrate metabolism | Sucrose phosphate synthase (MdSPS1) |
| Malate dehydrogenase (MdMDH) | |
| Malic enzyme (MdME) | |
| Aldose-6-phosphate reductase (MdA6PR) | |
| Sorbitol dehydrogenase(MdSDH1) | |
| Cell wall invertase (MdCWINV1) | |
| Salt overly sensitive (SOS) pathway | MdSOS1 |
| MdSOS2 | |
| MdSOS3 | |
| MdHKT1 | |
| MdNHX1 | |
| Nitrate transporters | CsNRT1.1 |
| CsNR1. | |
| Antioxidant genes | cAPX |
| cGR | |
| MDHAR | |
| DHAR-1 | |
| Aquaporin gene | OsPIP1 |
| OsPIP2 | |
| OsPIP3 | |
| IAA | IAA oxidase genes |
However, there are numerous vital issues that need to be elucidated in the future. Genes involved in dopamine biosynthesis and metabolism pathway in plants remain to be further clarified. Many studies have focused on the application of exogenous dopamine to plants and the effects of increasing endogenous dopamine through transgenic methods need to be more thoroughly explored. In addition, the study of catecholamine receptors is helpful to reveal the mechanism of it at the molecular level. Dopamine receptors in plants have not been reported. However, many experiments have shown the existence of plant catecholamine receptors. Verelst et al. (2004) have identified a class of DoH-CB proteins in plants that can regulate the activity of catecholamines.76 DoH-CB protein can bind to dopamine through the induction of auxin. It is speculated that DoH-CB protein may be the receptor of plant catecholamines, and the binding between them is induced by auxin.77 To conclude, dopamine increase plant stress resistance is a new field that needs further study, but may provide useful clues for the cultivation of new plant varieties resistant to stress.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31972389), the earmarked fund for the China Agricultural Research System (CARS-27), and Tang Scholar.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Kulma A, Szopa J.. Catecholamines are active compounds in plants. Plant Sci. 2007;172(3):1–8. doi: 10.1016/j.plantsci.2006.10.013. [DOI] [Google Scholar]
- 2.Howe MW, Dombeck DA.. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 2016;535(7613):505–510. doi: 10.1038/nature18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Che T, Levit A, Shoichet BK, Wacker D, Roth BL.. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature. 2018;555:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etienne AC, Peter JH, Andrea NB, Aparna S, Nicholas GC, Christine S, Thorvald FA, Ulrik G, Jeremy V, James SS, et al. Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine. 2015;2:135–146. doi: 10.1016/j.ebiom.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denby K, Gehring C. Engineering drought and salinity tolerance in plants: lessons from genome-wide expression profiling in Arabidopsis. Trends Biotechnol. 2005;23:547–552. doi: 10.1016/j.tibtech.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Zandalinas SI, Yosef F, Devireddy AR, Soham S, Azad RK, Ron M. Systemic signaling during abiotic stress combination in plants. Proc Natl Acad Sci U S. 2020;117:13810–13820. doi: 10.1073/pnas.2005077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants. 2019;8:34–41. doi: 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savvides A, Ali S, Tester M, Fotopoulos V. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci. 2016;21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Gao T, Zhang Z, Liu X, Wu Q, Chen Q, Liu Q, Nocker SV, Ma F, Li C. Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol Biochem. 2020;148(2020):260–272. doi: 10.1016/j.plaphy.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Endress R, Jäger A, Kreis W. Catecholamine biosynthesis dependent on the dark in betacyanin-forming portulaca callus. J Plant Physiol. 1984;115(4):291–295. doi: 10.1016/S0176-1617(84)80101-7. [DOI] [PubMed] [Google Scholar]
- 11.Liang B, Li C, Ma C, Wei Z, Wang Q, Huang D. Dopamine alleviates nutrient deficiency-induced stress in malus hupehensis. Plant Physiol Biochem. 2017;119:346. doi: 10.1016/j.plaphy.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Sun XK, Chang C, Jia DF, Wei ZW, Li CY, Ma FW. Dopamine alleviates salt-induced stress in Malus hupehensis. Physiol Plant. 2015;153(4):584–602. doi: 10.1111/ppl.12264. [DOI] [PubMed] [Google Scholar]
- 13.Liang B, Gao T, Zhao Q, Ma C, Chen Q, Wei Z, Li C, Ma F. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front Plant Sci. 2018;9:755. doi: 10.3389/fpls.2018.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao XY, Li YX, Zhang XZ, Liu CL, Liang W, Li C, Ma FW, Li CY. Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants. 2019;8:580. doi: 10.3390/plants8120580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura M. Fluorescence histochemical study on serotonin and catecholamine in some plants. Jpn J Clin Pharmacol. 1968;18(2):162–168. doi: 10.1254/jjp.18.162. [DOI] [PubMed] [Google Scholar]
- 16.Feldman JM, Lee EM, Castleberry CA. Catecholamine and serotonin content of foods: effect on urinary excretion of homovanillic and 5-hydroxyindoleacetic acid. J Am Diet Assoc. 1987;87:1031–1035. [PubMed] [Google Scholar]
- 17.Gao T, Liu X, Shan L, Wu Q, Liu Y, Zhang Z, Ma F, Li C. Dopamine and arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ Exp Bot. 2020;178:104159. doi: 10.1016/j.envexpbot.2020.104159. [DOI] [Google Scholar]
- 18.Kong KH, Lee JL, Park HJ, Cho SH. Purification and characterization of the tyrosinase isozymes of pine needles. IUBMB Life. 1998;45:717–724. doi: 10.1080/15216549800203122. [DOI] [PubMed] [Google Scholar]
- 19.Steiner U, Schliemann W, Strack D. Assay for tyrosine hydroxylation activity of tyrosinase from betalain-forming plants and cell cultures. Anal Biochem. 1996;238:72–75. doi: 10.1006/abio.1996.0253. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsu I, Sudo Y, Nagatsu T. Tyrosine hydroxylation in the banana plant. Enzymologia. 1972;43:25. [PubMed] [Google Scholar]
- 21.Lundström J. Biosynthesis of mescaline and tetrahydroisoquinoline alkaloids in Lophophora williamsii (Lem.). Coult Acta Pharm Suec. 1971;8:261–274. [PubMed] [Google Scholar]
- 22.Paul AG. Biosynthesis of peyote alkaloids. Llyodia. 1973;36:36–45. [PubMed] [Google Scholar]
- 23.Świȩdrych A, Lorenc-Kukuła K, Skirycz A, Szopa J. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol Biochem. 2004;42:593–600. doi: 10.1016/j.plaphy.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Kamisaka S. Requirement of cotyledons for gibberellic acid-induced hypocotyl elongation in lettuce seedlings. Isolation of the cotyledon factor active in enhancing the effect of gibberellic acid. Plant Cell Physiol. 1973;14:747–755. [Google Scholar]
- 25.Kamisaka S. Catecholamine stimulation of the gibberellin action that induces lettuce hypocotyl elongation. Plant Cell Physiol. 1979;20(7):1199–1207. doi: 10.1093/oxfordjournals.pcp.a075919. [DOI] [Google Scholar]
- 26.Elstner EF, Konze JR, Selman BR, Stoffer C. Ethylene formation in sugar beet leaves: evidence for the involvement of 3-hydroxytyramine and phenoloxidase after wounding. Plant Physiol. 1976;58(2):163–168. doi: 10.1104/pp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taiz L, Zeiger E. Fisiologia vegetal. In: Taiz L, Zeiger E, editor. Porto Alegre: Artmed. 2009:738–773. [Google Scholar]
- 28.Kuklin A, Conger B. Enhancement of somatic embryogenesis in orchardgrass leaf cultures by epinephrine. Plant Cell Rep. 1995;14:641–644. doi: 10.1007/BF00232729. [DOI] [PubMed] [Google Scholar]
- 29.Goldacre PL, Galston AW, Weintraub RL. The effect of substituted phenols on the activity of the indoleacetic acid oxidase of peas. Arch Biochem Biophys. 1953;43:358–373. doi: 10.1016/0003-9861(53)90130-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee TT, Starratt AN, Jevnikar JJ. Regulation of enzymic oxidation indole-3-acetic acid by phenols: structure-activity relationships. Phytochemistry. 1982;21:517–523. doi: 10.1016/0031-9422(82)83132-4. [DOI] [Google Scholar]
- 31.Guidotti BB, Gomes BR, de Cássia Siqueira-soares R, Soares AR, Ferrarese-Filho O. The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signaling Behav. 2013;8(9):e25477. doi: 10.4161/psb.25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiedrych A, Stachowiak J, Szopa J. The catecholamine potentiates starch mobilization in transgenic potato tubers. Plant Physiol Biochem. 2004;42:103–109. [DOI] [PubMed] [Google Scholar]
- 33.Skirycz A, Swiedrych A, Szopa J. Expression of human dopamine receptor in potato (Solanum tuberosum) results in altered tuber carbon metabolism. BMC Plant Biol. 2005;5:1. doi: 10.1186/1471-2229-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen JF. Superoxide as an obligatory, catalytic intermediate in photosynthetic reduction of oxygen by adrenaline and dopamine. Antioxid Redox Signal. 2003;5(1):7–14. doi: 10.1089/152308603321223496. [DOI] [PubMed] [Google Scholar]
- 35.Khurana J. Role of catecholamines in promotion of flowering in a short-day Duckweed, Lemna paucicostata 6746. Plant Physiol. 1987;85(1):10–12. doi: 10.1104/pp.85.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protacio CM, Dai YR, Lewis EF, Flores HE. Growth stimulation by catecholamines in plant tissue/organ cultures. Plant Physiol. 1992;98:89–96. doi: 10.1104/pp.98.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brugnoli E, Björkman O. Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non–stomatal components of photosynthesis and dissipation of excess light energy. Planta. 1992;187:335–347. doi: 10.1007/BF00195657. [DOI] [PubMed] [Google Scholar]
- 38.Farooq M, Wahid A, Lee DJ, Ito O, Siddique KH. Advances in drought resistance of rice. Criti Rev Plant Sci. 2009;28:199–217. doi: 10.1080/07352680902952173. [DOI] [Google Scholar]
- 39.Yamane K, Hayakawa K, Kawasaki M, Taniguchi M, Miyake H. Bundle sheath chloroplasts of rice are more sensitive to drought stress than mesophyll chloroplasts. J Plant Physiol. 2003;160:1319–1327. doi: 10.1078/0176-1617-01180. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian K, Santhanakrishnan P, Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci Hortic. 2006;107:245–253. doi: 10.1016/j.scienta.2005.07.006. [DOI] [Google Scholar]
- 41.Ludlow M, Muchow R. A critical evaluation of traits for improving crop yields in water-limited environments. Adv Agron. 1990;43:107153. [Google Scholar]
- 42.Yanosky TM. Principles of soil and plant-water relations. J Environ Qual. 2005;34(4):1452–a. doi: 10.2134/jeq2005.0006br. [DOI] [Google Scholar]
- 43.Lehmann T, Pollmann S. Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana. FEBS Lett. 2009;583:1895–1900. doi: 10.1016/j.febslet.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Zhou S, Li M, Guan Q, Liu F, Zhang S, Chen W. Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in malus. Plant Sci. 2015;236:44–60. doi: 10.1016/j.plantsci.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Bai T, Li C, Li C, Liang D, Ma F. Contrasting hypoxia tolerance and adaptation in Malus species is linked to differences in stomatal behavior and photosynthesis. Physiol Plant. 2013;147:514–523. doi: 10.1111/j.1399-3054.2012.01683.x. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman L, Dacosta M, Ebdon JS, Zhao J. Effects of drought preconditioning on freezing tolerance of perennial ryegrass. Environ Exp Bot. 2012;79:11–20. doi: 10.1016/j.envexpbot.2012.01.002. [DOI] [Google Scholar]
- 47.Sheikh-Mohamadi MH, Etemadi N, Arab MM, Aalifar M, Arab M. Physiological and ascorbate-glutathione pathway-related genes responses under drought and heat stress in crested wheatgrass. Sci Hortic. 2018;242:195–206. doi: 10.1016/j.scienta.2018.07.037. [DOI] [Google Scholar]
- 48.Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J Agr Food Chem. 2000;48:844–848. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- 49.Rosel MA, Mosca L, Foppoli C, Blarzino C, Coccia R. Lipoxygenase-catalyzed oxidation of 5-S-substituted catecholamines. Melanoma Res. 1994;200:344–350. [DOI] [PubMed] [Google Scholar]
- 50.Romero-Aranda R, Soria T, Cuartero J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001;160:265–272. doi: 10.1016/S0168-9452(00)00388-5. [DOI] [PubMed] [Google Scholar]
- 51.Flexas J, Medrano H. Energy dissipation in C3 plants under drought. Funct Plant Biol. 2002;29:1209–1215. doi: 10.1071/FP02015. [DOI] [PubMed] [Google Scholar]
- 52.Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotox Environl Safe. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Lan GP, Jiao CJ, Wang GQ, Sun YH, Yan S. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci Hortic. 2020;260:108790. doi: 10.1016/j.scienta.2019.108790. [DOI] [Google Scholar]
- 54.Geissler N, Huchzermeyer B, Koyro HW. Effects of salt stress on photosynthesis under ambient and elevated atmospheric CO2 concentration. In: Ahmad P, Azooz MM, Prasad MNV, editors. Salt stress in plants. New York (NY): Springer. 2013;377–413. [Google Scholar]
- 55.Abdelkader AF, El-khawas S, El-Sherif NASE, Hassanein RA, Emam MA, Hassan RE. Expression of aquaporin gene (OsPIP1-3) in salt-stressed rice (Oryzasativa L.) plants pre-treated with the neurotransmitter (dopamine). Plant Omics. 2012;5:532–541. [Google Scholar]
- 56.Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch–Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci U S A. 2007;104:15270–15275. doi: 10.1073/pnas.0707294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992;33:1215–1223. [Google Scholar]
- 58.Mittova V, Tal M, Volokita M, Guy M. Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt-tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiol Plant. 2002;115:393–400. doi: 10.1034/j.1399-3054.2002.1150309.x. [DOI] [PubMed] [Google Scholar]
- 59.Mittova V, Tal M, Volokita M, Guy M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 2003;26:845–856. doi: 10.1046/j.1365-3040.2003.01016.x. [DOI] [PubMed] [Google Scholar]
- 60.Selote DS, Khanna-Chopra R. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol Plant. 2006;127:494–506. doi: 10.1111/j.1399-3054.2006.00678.x. [DOI] [Google Scholar]
- 61.Salazar-Parra C, Aguirreolea J, Sánchez-Díaz M, Irigoyen JJ, Morales F. Climate change (elevated CO2, elevated temperature and moderate drought) triggers the antioxidant enzymes’ response of grapevine cv. Tempranillo, avoiding oxidative damage. Physiol Plant. 2012;144:99–110. doi: 10.1111/j.1399-3054.2011.01524.x. [DOI] [PubMed] [Google Scholar]
- 62.Willson KG, Perantoni AN, Berry ZC, Eicholtz MI, Tamukong YB, Yarwood SA, Baldwin AH. Influences of reduced iron and magnesium on growth and photosynthetic performance of Phragmites australis subsp. americanus (North American common reed). Aquat Bot. 2017;137:30–38. doi: 10.1016/j.aquabot.2016.11.005. [DOI] [Google Scholar]
- 63.Gruber BD, Giehl RFH, Friedel S, von Wiren N. Plasticity of the arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013;163(1):161–179. doi: 10.1104/pp.113.218453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song X, Wan F, Chang X, Zhang J, Sun M, Liu Y. Effects of nutrient deficiency on root morphology and nutrient allocation in pistacia chinensis bunge seedlings. Forests. 2019;10:1035. [Google Scholar]
- 65.Lynch JP. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 2011;156(3):1041–1049. doi: 10.1104/pp.111.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annu Rev Plant Biol. 2007;58:93–113. doi: 10.1146/annurev.arplant.58.032806.104006. [DOI] [PubMed] [Google Scholar]
- 67.Sirohi G, Pandey BK, Deveshwar P, Giri J. Emerging trends in epigenetic regulation of nutrient deficiency response in plants. Mol Biotechnol. 2016;58(3):159–171. doi: 10.1007/s12033-016-9919-0. [DOI] [PubMed] [Google Scholar]
- 68.Facchini PJ, Johnson AG, Poupart J, Luca V. Uncoupled defense gene expression and antimicrobial alkaloid accumulation in elicited opium poppy cell cultures. Plant Physiol. 1996;111(3):687–697. doi: 10.1104/pp.111.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negrel J, Javelle F, Paynot M. Wound-induced tyramine hydroxycinnamoyl transferase in Potato (Solanum tuberosum) tuber discs. J Plant Physiol. 1993;142(5):518–524. doi: 10.1016/S0176-1617(11)80392-5. [DOI] [Google Scholar]
- 70.Facchini PJ, Yu M, Penzes-Yost C. Decreased cell wall digestibility in canola transformed with chimeric tyrosine decarboxylase genes from opium poppy. Plant Physiol. 1999;120:653–663. doi: 10.1104/pp.120.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SU, Johnson AG, Penzes–Yost C, Facchini PJ. Analysis of promoters from tyrosine/dihydroxyphenylalanine decarboxylase and berberine bridge enzyme genes involved in benzylisoquinoline alkaloid biosynthesis in opium poppy. Plant Mol Biol. 1999;40(1):121–131. doi: 10.1023/A:1026433112119. [DOI] [PubMed] [Google Scholar]
- 72.Negrel J, Javelle F. L-Tyrosine beta-naphthylamide is a potent competitive inhibitor of tyramine N-(hydroxycinnamoyl) transferase in vitro. Phytochemistry. 2001;56:523–527. doi: 10.1016/S0031-9422(00)00427-1. [DOI] [PubMed] [Google Scholar]
- 73.Yogendra KN, Dhokane D, Kushalappa AC, Sarmiento F, Rodriguez E, Mosquera T. StWRKY8 transcription factor regulates benzylisoquinoline alkaloid pathway in potato conferring resistance to late blight. Plant Sci. 2016;256:208–216. doi: 10.1016/j.plantsci.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Landtag J, Baumert A, Degenkolb T, Jürgen S, Rosahl S. Accumulation of tyrosol glucoside in transgenic potato plants expressing a parsley tyrosine decarboxylase. Phytochemistry. 2002;60:683–689. [DOI] [PubMed] [Google Scholar]
- 75.Trujillo-Villanueva K, Rubio-Piña J, Monforte-González M, Ramírez-Benítez E, Vázquez-Flota F. The sequential exposure to jasmonate, salicylic acid and yeast extract promotes sanguinarine accumulation in Argemone mexicana cell cultures. Biotechnol Lett. 2012;34:379–385. doi: 10.1007/s10529-011-0770-x. [DOI] [PubMed] [Google Scholar]
- 76.Verelst W, Asard H. Analysis of an Arabidopsis thaliana protein family, structurally related to cytochromes b561 and potentially involved in catecholamine biochemistry in plants. J Plant Physiol. 2004;161:175–181. doi: 10.1078/0176-1617-01064. [DOI] [PubMed] [Google Scholar]
- 77.Zhang CL, Li YF. Study on catecholamine in plant. Chemistry of Life. 2008;28:418–421. (In chinese). [Google Scholar]


