Figure 1.
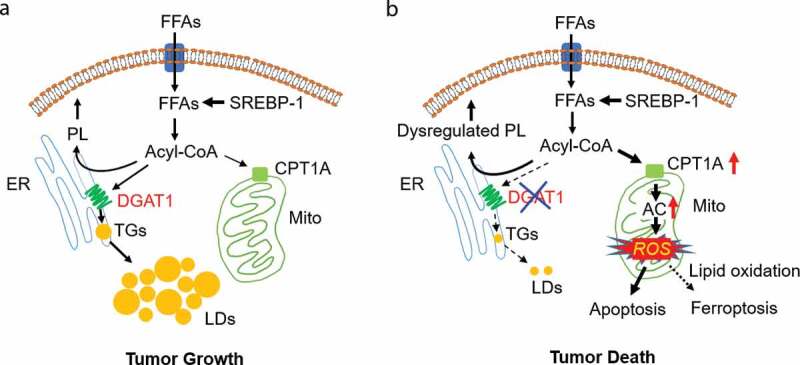
DGAT1: a promising metabolic target for cancer therapy. (a, b) Schematic model illustrating the function of diacylglycerol O-acyltransferase 1 (DGAT1) in regulating lipid homeostasis and lipotoxicity resulting from DGAT1 inhibition in tumor cells. Tumor cells acquire sufficient free fatty acids (FFA) for rapid growth via activation of sterol regulatory element-binding protein 1 (SREBP-1)-regulated de novo synthesis. In addition, FFA uptake also contribute to the pool of FFAs in tumor cells. FFA need to bind to coenzyme A to form acyl-CoA for subsequent synthesis of phospholipids (PL) in the endoplasmic reticulum (ER), or for shuttling to mitochondria for energy production. To prevent excess FFA from inducing toxicity, tumor cells upregulate the expression of DGAT1 to convert excess FFA to triglycerides (TGs) that form lipid droplets (LDs) for storage in the cytosol of cells (a). Targeting DGAT1 will cause the dysregulation of lipid homeostasis and the increase of carnitine palmitoyltransferase 1A (CPT1A) expression to convert excess FFA into acylcarnitines (AC) that enter into mitochondria to generate high levels of reactive oxygen species (ROS). ROS damage mitochondria, trigger apoptosis and can also increase lipid oxidation to induce ferroptosis, together killing tumor cells (b)
