Figure 20.
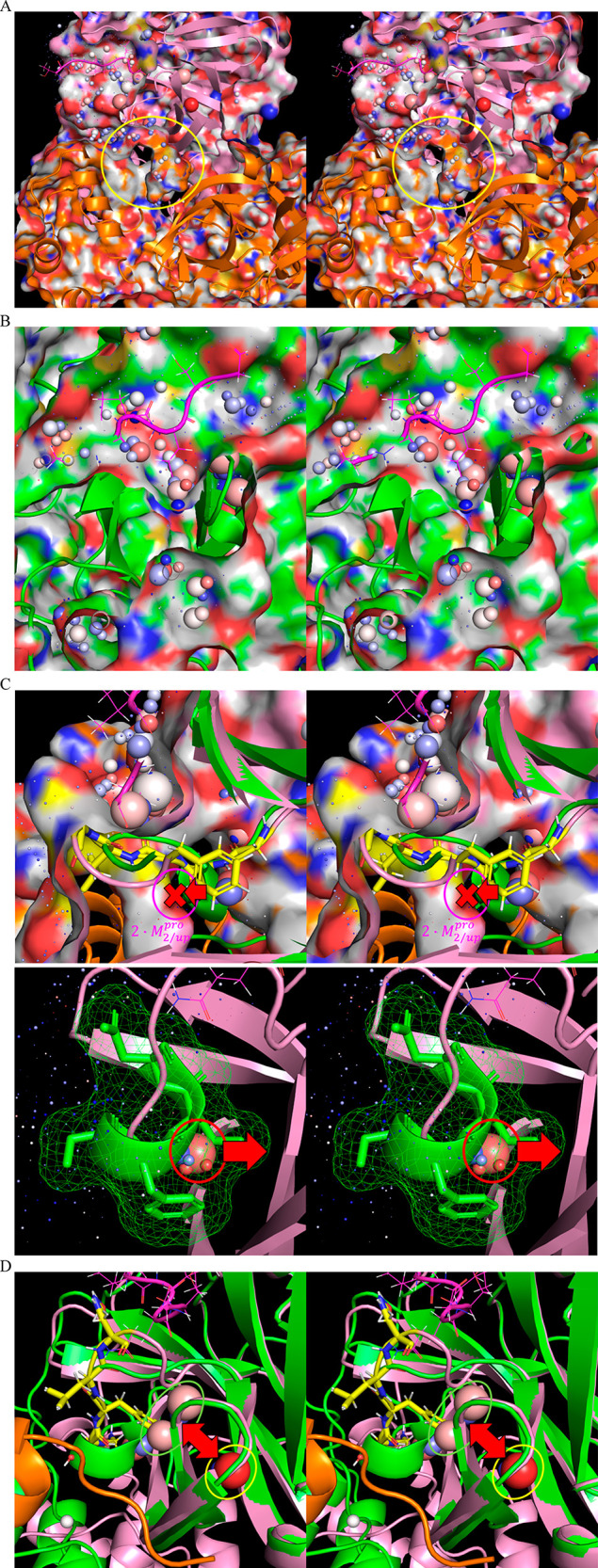
(A) Stereo view of the time-averaged 2·M2/uppro structure (6M03) (clipped through the external protein surface) showing channel 3, which resides adjacent to the rising stem of the m-shaped loop (circled in yellow), the lining of which is contributed largely by the opposite monomer (orange). (B) Stereo view of the same region in the time-averaged M1/down structure (2QCY) (clipped through the external protein surface), noting the absence of channel 3 in this state. (C) Stereo view of the time-averaged M1/downpro structure overlaid on the time-averaged 2·M2/up structure (green and pink, respectively), depicting the putative solvation free energy transduction mechanism driving the down and up states of the m-shaped loop. Top: Formation of channel 3 in the 2·M2/uppro state drives the rising stem of the loop into the up conformation due the high cost of desolvating the channel by the 310 helical turn (denoted by the red X). Bottom: Conversely, 310 helix formation is promoted in the M1/down state via the expulsion of a trapped water (green arrow), together with several H-bond depleted waters on the external protein surface (yellow circle) that are present in the 2·M2/uppro state. (D) Stereo view of the time-averaged M1/down structure (2QCY) overlaid on the time-averaged 2·M2/uppro2·M2/up structure (2Q6G), showing the UHOVs in the respective structures (circled in green and pink, respectively). Conservation of these unfavorable UHOVs (likely representing a single water molecule) in both states (the shifted positioning denoted by the red arrow) suggests that they contribute to the local instability and rearrangeability of the m-shaped loop.
