Table 3.
Antifiloviral Activity of 4-(Aminomethyl)benzamides 41–50, 52-54and 5-(Aminomethyl)thiophenecarboxamide 55.
| N | Structure | Ebola pseudovirus A549 cells | Marburg pseudovirus A549 cells | |||
|---|---|---|---|---|---|---|
| % inhibition | EC50a (μM) | SIb | % inhibition | EC50a (μM) | ||
 | ||||||
| 41 | R1 = R2 = H, R3 = 5-CO2Me | 41.7 | - | 51.2 | - | |
| 42 | R1 = R2 = H, R3 = 5-CF3 | 56.4 | - | 49.6 | - | |
| 43 | R1 = R2 = H, R3 = 6-CF3 | 83.7 | 1.68 ± 0.43 | 38 | 59.2 | 1.46 ± 0.41 |
| 44 | R1 = R2 = H, R3 = 6-Cl | 81.5 | 2.22 ± 0.39 | 14 | 42.4 | 2.31 ± 0.37 |
| 45 | R1 = R2 = H, R3 = 6-F | 20.1 | - | 13.5 | - | |
| 46 | R1 = H, R2 = di-Me, R3 = H | 87.3 | 2.22 ± 1.02 | 33 | 21.4 | 2.90 ± 0.87 |
| 47 | R1 = H, R2 = cyclo-Pr, R3 = H | 74.5 | - | 34.4 | - | |
| 48 | R1 = Me, R2 = H, R3 = 6-CF3 | 95.6 | 1.56 ± 0.69 | 21 | 76.9 | 1.73 ± 0.88 |
| 49 | R1 = Me, R2 = H, R3 = 5-t-Bu | 99.8 | 2.05 ± 0.33 | 9 | 98.1 | 3.09 ± 0.53 |
| 50 | R1 = Me, R2 = t-Bu, R3 = H | 99.9 | 0.16 ± 0.07 | 224 | 92.8 | 3.18 ± 1.33 |
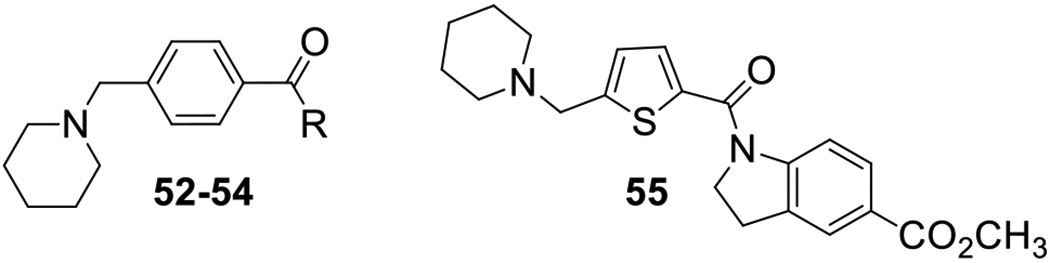 | ||||||
| 52 | 5.4 | - | 9.4 | - | ||
| 53 | 25.1 | - | 0 | - | ||
| 54 |  |
0 | - | 0 | - | |
| 55 | 38.9 | - | 0 | - | ||
| toremifene | 0.09 ± 0.04 | 299 | ||||
Dose-response studies were conducted to determine EC50 values for those compounds that showed more than 75% inhibition at 12.5 uM concentration; EC50 values were calculated by four-parameter dose-response curve-fitting in GraphPad. Results are from three replicates. Percent inhibition errors are estimated to be <10%; EC50 data are presented as mean ± SD.
Selectivity index is the ratio of CC50/EC50, where CC50 is cytotoxicity assessed by utilizing the “CellTiter 96 aqueous nonradioactive cell proliferation assay” (Promega, Madison, WI).
