Supplemental Digital Content is available in the text.
Keywords: acute respiratory failure, coronavirus disease 2019, nitric oxide, rescue therapy
Objectives:
Treatment options are limited for patients with respiratory failure due to coronavirus disease 2019. Conventional oxygen therapy and awake proning are options, but the use of high-flow nasal cannula and continuous positive airway pressure are controversial. There is an urgent need for effective rescue therapies. Our aim is to evaluate the role of inhaled nitric oxide 160 ppm as a possible rescue therapy in nonintubated coronavirus disease 2019 patients.
Design:
Retrospective evaluation of coronavirus disease 2019 patients in respiratory distress receiving nitric oxide gas as rescue therapy.
Setting:
Massachusetts General Hospital, between March 18, 2020, and May 20, 2020, during the local coronavirus disease 2019 surge.
Patients:
Coronavirus disease 2019 patients at high risk for acute hypoxemic respiratory failure with worsening symptoms despite use of supplemental oxygen and/or awake proning.
Interventions:
Patients received nitric oxide at concentrations of 160 ppm for 30 minutes twice per day via a face mask until resolution of symptoms, discharge, intubation, or the transition to comfort measures only.
Measurements and Main Results:
Between March 18, 2020, and May 20, 2020, five patients received nitric oxide inhalation as a rescue therapy for coronavirus disease 2019 at Massachusetts General Hospital. All received at least one dosage. The three patients that received multiple treatments (ranging from five to nine) survived and were discharged home. Maximum methemoglobin concentration after 30 minutes of breathing nitric oxide was 2.0% (1.7–2.3%). Nitrogen dioxide was below 2 ppm. No changes in mean arterial pressure or heart rate were observed during or after nitric oxide treatment. Oxygenation and the respiratory rate remained stable during and after nitric oxide treatments. For two patients, inflammatory marker data were available and demonstrate a reduction or a cessation of escalation after nitric oxide treatment.
Conclusions:
Nitric oxide at 160 ppm may be an effective adjuvant rescue therapy for patients with coronavirus disease 2019.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to coronavirus disease 2019 (COVID-19) and has various clinical manifestations, ranging from asymptomatic, or mild cold-like symptoms, to critical life-threatening conditions, including respiratory failure, septic shock, and/or multiple organ dysfunction (1). More than 75% of hospitalized COVID-19 patients require supplemental oxygen, and 17% to 35% of hospitalized patients are treated in an ICU, predominantly due to respiratory failure (1). Targeting an improvement in oxygenation, first-line treatment strategies include awake proning and supplemental oxygen (2). For additional oxygen delivery, nasal cannulas and nonrebreather masks are commonly used. The use of continuous positive airway pressure (CPAP) or high-flow nasal cannula (HFNC) and other noninvasive ventilation strategies is controversial in patients with acute hypoxic respiratory failure, due to concerns of the increased risk of exposing healthcare workers to infectious aerosols (3). If respiratory status worsens, initiation of invasive mechanical ventilation may be required to prevent life-threatening hypoxemia and additional organ damage (2). However, many COVID-19 patients are elderly with multiple comorbidities and maintain “do not resuscitate” and/or “do not intubate” (DNI) orders, further limiting treatment options. For patients with imminent respiratory failure, independent of patients’ healthcare directive status, we offer inhaled nitric oxide (NO) as a rescue therapy when requested by the responsible clinicians at our institution. During the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) outbreak in 2003, NO improved arterial oxygenation and recovery rates in a small group of critically ill patients with coronavirus-associated pneumonia (4). The extent of the benefit following NO inhalation was not exclusively explained by the improvement in oxygenation related to enhanced ventilatory/perfusion matching, and the authors hypothesized a potential anti-inflammatory and antiviral effect of NO.
Due to the genetic similarities of the two SARS coronaviruses and the promising experience using NO during the SARS-CoV-1 outbreak, multiple trials have been initiated to evaluate the safety and effectiveness of NO against COVID-19 (5).
Here, we present a case series of spontaneous breathing severe COVID-19 adult patients that, due to clinically deteriorating respiratory conditions despite best practice, received 160 parts per million (ppm) NO for 30 minutes twice per day as a rescue therapy.
MATERIALS AND METHODS
Given our long history of using inhaled NO for acute respiratory distress syndrome (ARDS) and pulmonary hypertension (6) and initiating clinical trials investigating the efficacy of NO gas for COVID-19 patients (5), we offered inhaled NO therapy as an innovative rescue therapy for COVID-19 patients with rapidly progressive hypoxemic respiratory failure. These particular patients were not considered eligible for other rescue therapies within or outside clinical trials.
Patients who received NO gas treatment as a rescue therapy, requested by managing clinicians, were retrospectively reviewed by the study team. Demographic and clinical data (cardiopulmonary function and laboratory results) were recorded. The oxygenation was expressed as peripheral oxygen saturation (Spo2)/Fio2 as a surrogate marker of Pao2/Fio2 (7). NO was delivered by our study team at 160 ppm twice a day for 30 minutes with a custom-made delivery device for spontaneously breathing patients using a well-fitting mask (Fig. 1A). Currently, there are no guidelines for the use of NO for COVID-19 infections. For the treatment of bacterial pneumonia, NO of 160 ppm was the lowest dose effective in a dose-response curve and is a dose that is clinically feasible and does not lead to excessive methemoglobinemia (8). The system to deliver NO gas was able to maintain the level of nitrogen dioxide, a toxic byproduct of NO gas, below the safety limit of 2 ppm. Methemoglobin, a type of hemoglobin derived by the reaction between NO and hemoglobin, was monitored noninvasively using CO-oximetry (Radical 7; Masimo, Irvine, CA) (Fig. 1B) (9). The local Institutional Review Board (number 2020P001735) approved collection and analysis of the data for this retrospective study.
Figure 1.
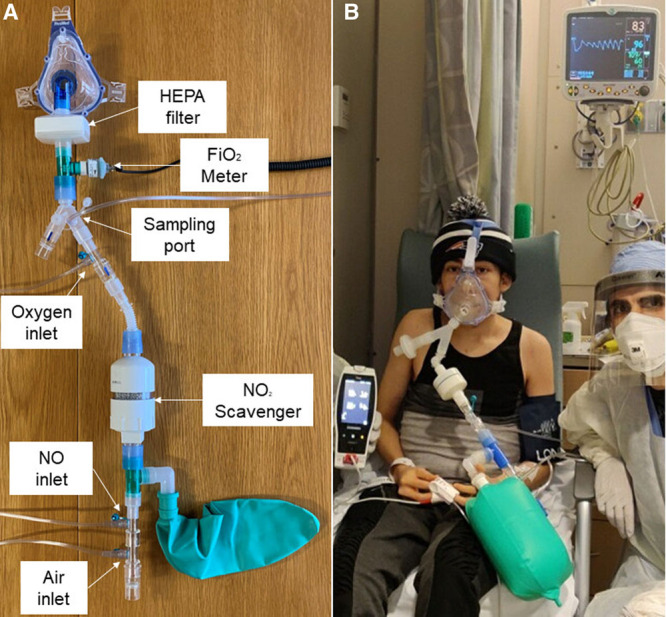
Nitric oxide (NO) delivery device and administration. A, NO delivery device build from respiratory care components for spontaneously breathing patients. Main components are tubings for medical air, NO in nitrogen and oxygen, sampling lines for oxygen content, NO and nitrogen dioxide (No2) concentration, one-way valves, high efficiency particulate air (HEPA) filter, scrubber with soda lime, and mask. B, NO administration in an adult patient using one of our delivery devices.
RESULTS
Between March 18, 2020, and May 20, 2020, our response team was called upon to provide NO inhalation as rescue therapy for five patients at Massachusetts General Hospital. All patients had multiple comorbidities. None of these patients received remdesivir due to a lack of availability or a clinical contraindication. The patients’ clinical characteristics are shown in Table 1. Patients 1, 2, and 3 received between five to nine treatments in total. The median methemoglobin level at baseline was 0.3% (0.1–0.7%), and the peak value after 30 minutes was 2.0% (1.7–2.3%). Average peaks of methemoglobin of each patient are displayed in Table 1. Five minutes after cessation of the treatment, methemoglobin was 1.6% (1.0–2.0%). The three patients subjectively reported an improvement in breathing. The patients rested comfortably and, at times, fell asleep during treatment.
TABLE 1.
Baseline Characteristics and Treatment Course
| General | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age, yr | 57 | 70 | 60 | 83 | 73 |
| Body mass index, kg/m2 | 24.4 | 22.9 | 28.0 | 30.3 | 35.9 |
| Underlying chronic disease/conditions | OSAS, chronic sinusitis, liver function tests elevation, gastroesophageal reflux disease, dysgammaglobulinemia, hypothyroidism | Hypertension, coronary artery disease, COPD, rectal cancer | Hypertension, thalassemia, psoriasis | Hypertension, chronic kidney disease polymyalgia rheumatica | COPD, OSAS, lung cancer, diabetes mellitus type II, hypothyroidism, dementia |
| Code | Full | DNR/DNI | Full | DNR/DNI | DNR/DNI |
| Days from onset of symptoms until therapy start/evaluation | 13 | 14 | 9 | 5 | 13 |
| Days of severe acute respiratory syndrome coronavirus 2 positivity at admission | 0 | 9 | 8 | 0 | 0 |
| Disease severity by first NO administration | Critical | Critical | Critical | Critical | Critical |
| Respiratory rate > 30/min | No | No | Yes | Yes | Yes |
| Arterial oxygen saturation < 93% | Yes | Yes | Yes | Yes | Yes |
| Lung infiltrates > 50% | Yes | NA | Yes | NA | Yes |
| Oxygen need before first treatment (L) | 3 | 4 | 6 | 10 | 12 |
| C-reactive protein (mg/L) | 287 | 71 | 184 | 297 | 71 |
| NO treatments | 9 | 8 | 5 | 1 | 1 |
| Methemoglobin (%), peak | 2.5 | 2.7 | 2.7 | 1.2a | < 3b |
| Outcome | Discharged | Discharged | Discharged | Deceased | Deceased |
COPD = chronic obstructive pulmonary disease, DNR/DNI = do not resuscitate/do not intubate, NA = not applicable, NO = nitric oxide, OSAS = obstructive sleep apnea syndrome.
aThe patient received only one treatment.
bThe patient received only one treatment and methemoglobin remained below 3%.
Baseline characteristics and course of patients with severe coronavirus disease 2019 treated with 160 parts per million NO.
Two patients (patient 4 and patient 5) presented with severe respiratory distress, near respiratory failure and unable to maintain peripheral oxygen saturation above 90%, despite high supplemental oxygen (10 and 12 L/min) before NO gas started. Both patients were transitioned to comfort measurements within 24 hours after initiation of the first, and only, NO treatment.
As shown in Figure 2, A and B, no changes in mean arterial pressure or heart rate were observed during and after NO treatment. The respiratory rate did not change during or after the treatment (Fig. 2C). Oxygenation, expressed as Spo2/Fio2, remained stable during and after the treatments, whether the patient was initially hypoxemic or not (Fig. 2D).
Figure 2.
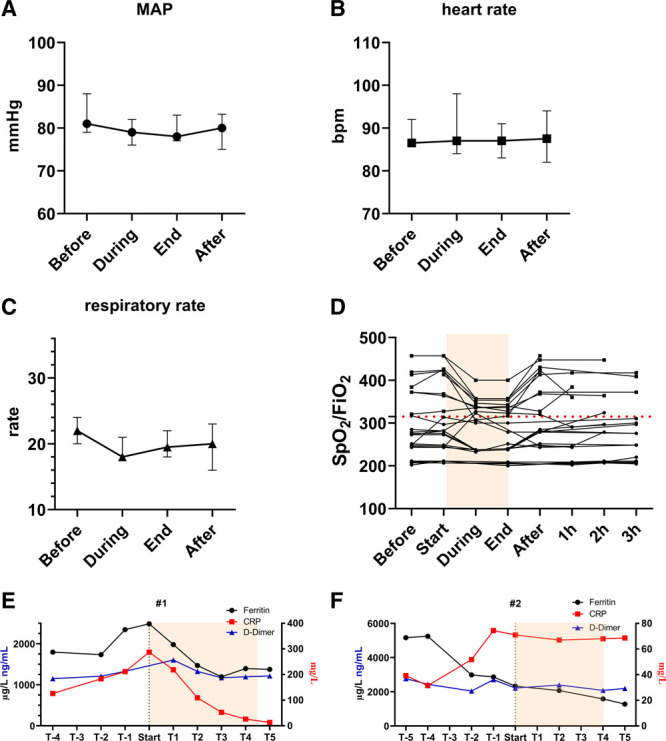
Vital variables and laboratory variables in severe coronavirus disease 2019 patients receiving nitric oxide (NO) treatment. Cardiopulmonary variables of patients receiving 160 parts per million NO with no negative effect on mean arterial pressure (MAP) (A), heart rate (B), or respiratory rate (C) (data shown as median and 95% CI). Oxygenation was not impaired and showed to be stable for normoxemic and hypoxemic cases (D). Course of laboratory results in patient 1 (E) and patient 2 (F). bpm = beats per minute, CRP = C-reactive protein, Spo2 = oxygen saturation.
For patient 1, inflammatory markers decreased after the initiation of NO treatment (Fig. 2E). In patient 2, the inflammatory markers appear to stop rising after start of NO (Fig. 2F). Inflammatory marker data from patient 3 are lacking.
The two patients who were transitioned to comfort measurements only ultimately died. Patient 3 was intubated for 9 days and was later safely extubated and discharged from the hospital. The length of stay for Patients 1, 2, and 3 was 9, 10, and 28 days, respectively.
DISCUSSION
In this study, we report the administration of 160 ppm NO as rescue therapy for nonintubated spontaneous breathing patients with severe COVID-19. Careful monitoring of vital signs did not demonstrate a systemic effect on blood pressure, heart rate, or respiratory rate, suggesting that a 30-minute treatment with 160 ppm does not adversely affect cardiopulmonary function in severe COVID-19 patients. Oxygenation during and after the treatment, and bedside monitoring of methemoglobin, showed that delivery of 160 ppm NO is well-tolerated, including treatment of patients with severe COVID-19. Patients tolerated the treatment exceptionally well and were often able to rest during inhalation, and their breathing effort was observed to decrease. We describe a possible anti-inflammatory property of NO, reflected in patients 1 and 2, but further data are needed to draw a definitive conclusion.
Recently, Parikh et al (10) published data describing the continuous administration of 30 ppm NO in spontaneously breathing patients with COVID-19. They observed an improvement in Spo2/Fio2 ratios in patients who did not require mechanical ventilation. In addition to using a lower dose of NO, we note that most of the patients included in their cohort received additional treatments targeting COVID-19, including an interleukin-6 receptor antagonist, hydroxychloroquine, and/or azithromycin.
All patients described here presented with comorbidities and had experienced worsening of symptoms over the days preceding initiation of NO therapy, with increased respiratory distress and increased supplemental oxygen demand despite the use of early proning. In noninfectious diseases, noninvasive ventilation, including CPAP or HFNC treatment, is a viable rescue treatment option, but at present, there is no evidence that using noninvasive ventilation leads to better outcomes in SARS-CoV-2 respiratory distress. In addition, noninvasive support is widely considered to generate infectious aerosols, leading to the spread of the virus, posing a high risk of exposure to healthcare workers (3). The respiratory guidelines at our institution restrict the use of HFNC and CPAP/bilevel positive airway pressure for the treatment of COVID-19 patients, further reducing supportive therapy options. In patients with multiple comorbidities, who are not eligible to be intubated and placed on mechanical ventilation (i.e., DNI), the lack of a viable rescue option will most likely result in death. For patients presenting with imminent respiratory failure, NO treatment might be an option, which can easily be combined with proning and antiviral treatment to slow the progression of COVID-19 infection and further clinical deterioration (6). Reducing hypoxia and enhancing bronchodilation may achieve some relief and alleviate suffering in a patient with COVID-19 who is otherwise comfort measures only.
NO inhalation at 10–80 ppm is Food and Drug Administration approved as a selective pulmonary vasodilator for hypoxic newborns with persistent pulmonary hypertension and is widely used off-label for adults with pulmonary hypertension and ARDS (6). During SARS-CoV-1, a direct effect of NO on the virus and the disease was postulated (4). In vitro studies confirmed antiviral properties of NO on SARS-CoV-1 in dose- and time-dependent manners (11). In addition, NO-mediated bronchodilation in the setting of obstructive lung disease, as well as its anti-inflammatory effect, make NO a strong treatment option for respiratory distress due to COVID-19 (5).
Remdesivir treatment is not recommended in adults with impaired renal function and/or an elevation of liver enzymes (12). For patients not eligible for remdesivir treatment or for patients on remdesivir with respiratory symptoms, other therapeutic interventions may be necessary to prevent respiratory failure. In addition, a shortage of remdesivir is possible, and alternative treatment options are needed (13).
There are limitations regarding the use of NO gas in spontaneously breathing COVID-19 patients. Breathing NO gas requires: 1) active participation by the patient in treatment and 2) employing the use of a well-defined clinical team administering the NO. Larger clinical trials are ongoing to further elucidate the efficacy of NO treatment in spontaneous breathing patients with COVID-19 (5).
CONCLUSIONS
We report that the administration of 160 ppm NO in nonintubated critically ill patients with COVID-19 is feasible and well-tolerated. The efficacy of NO as an adjunctive therapy for COVID-19 is being actively studied.
ACKNOWLEDGMENTS
We thank Drs. F. Ichinose, Y. Miyazaki, E. Marutani, and T. Ikeda for helping with nitric oxide treatment delivery.
Footnotes
Drs. Wiegand and Safaee Fakhr contributed equally to this article.
This study was supported by the Reginald Jenney Endowment Chair at Harvard Medical School to Dr. Berra, by Dr. Berra sundry funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital.
Dr. Wiegand receives a salary from the German Research Foundation number WI5162/2-1. Dr. Carroll receives funding from unitaid (an international organisation that invests in innovations to prevent, diagnose and treat HIV/AIDS, tuberculosis and malaria more quickly, affordably and effectively) as a principal investigator in a multi-institutional study working to decentralize laboratories to diagnose and treat tuberculosis in low-resource settings. Dr. Zapol is on the scientific advisory board of Third Pole, which has licensed patents on electric nitric oxide (NO) generation from Massachusetts General Hospital. Dr. Kacmarek is a consultant for Medtronic and Orange Med and has received research grants from Medtronic and Venner Medical (Dänischenhagen, Germany). Dr. Berra receives salary support from K23 HL128882/National Heart Lung and Blood Institute National Institutes of Health as principal investigator for his work on hemolysis and NO; he receives technologies and devices from inhaled NO (iNO) Therapeutics LLC, Praxair, and Masimo Corp; and he receives a grant from iNO Therapeutics LLC. Dr. Fakhr has disclosed that he does not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/ccejournal).
This study was conducted at Massachusetts General Hospital.
REFERENCES
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020; 324:782–793 [DOI] [PubMed] [Google Scholar]
- 2.Critical Care | Coronavirus Disease COVID-19. COVID-19 Treatment Guidelines. 2020. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed July 14, 2020
- 3.Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: Systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020; 67:1217–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Liu P, Gao H, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: A rescue trial in Beijing. Clin Infect Dis. 2004; 39:1531–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez RA, Berra L, Gladwin MT. Home nitric oxide therapy for COVID-19. Am J Respir Crit Care Med. 2020; 202:16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossaint R, Falke KJ, López F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993; 328:399–405 [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Wheeler AP, Bernard GR, et al. ; National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007; 132:410–417 [DOI] [PubMed] [Google Scholar]
- 8.Bogdanovski K, Chau T, Robinson CJ, et al. Antibacterial activity of high-dose nitric oxide against pulmonary Mycobacterium abscessus disease. Access Microbiol. 2020; 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni S, Morais CCA, Larson G, et al. Ideation and assessment of a nitric oxide delivery system for spontaneously breathing subjects. Nitric Oxide. 2020; 104–105:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh R, Wilson C, Weinberg J, et al. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther Adv Respir Dis. 2020; 14:1753466620933510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akerström S, Gunalan V, Keng CT, et al. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology. 2009; 395:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final Report. N Engl J Med. 2020NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White DB, Angus DC. A proposed lottery system to allocate scarce COVID-19 medications: Promoting fairness and generating knowledge. JAMA. 2020; 324:329–330 [DOI] [PubMed] [Google Scholar]


