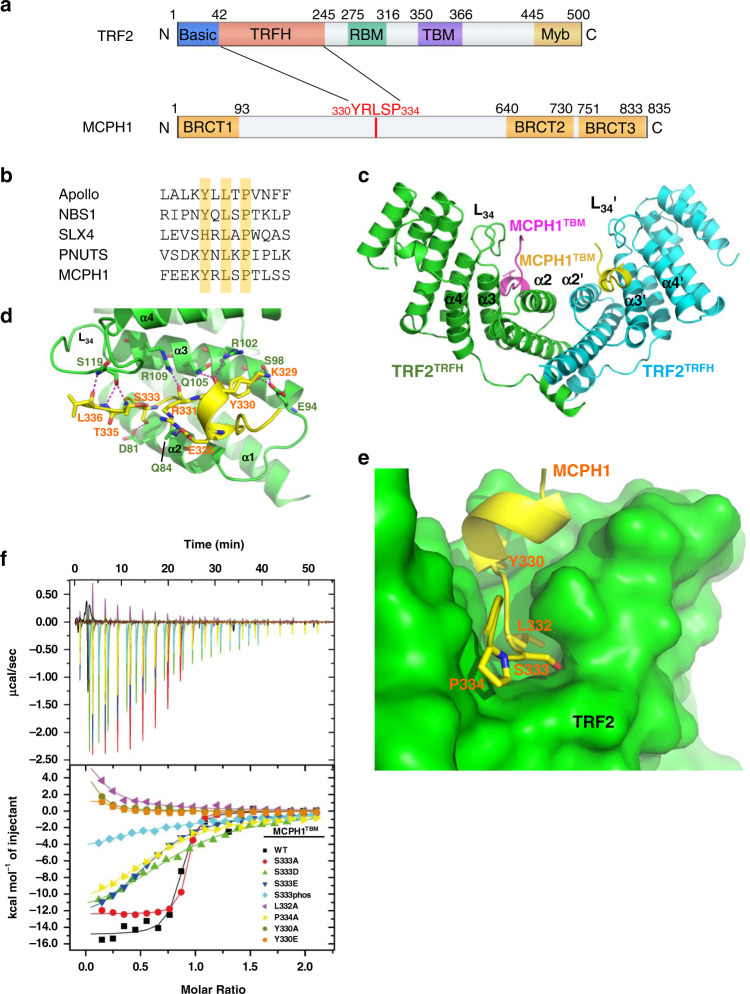
Fig. 1. Structure of the human TRF2TRFH–MCPH1TBM complex.
a Schematic representation of human TRF2 and MCPH1 domains, showing the interaction domains. b Comparison of MCPH1TBM sequence with those of known TRF2-interacting protein. The conserved amino acid Y/H-X-L-X-P consensus sequence is highlighted. c Dimeric TRF2–MCPH1 structure is shown in a ribbon representation (TRF2, green/cyan; MCPH1, magenta/yellow). d TRF2 and MCPH1 are depicted in green and yellow, respectively, and the residues involved in their interaction are shown. Hydrogen bonding: magenta dashed lines. e MCPH1TBM (in yellow) is buried inside a hydrophobic pocket formed by TRF2 helices α2 and α3 (in green). f ITC measurement of the interactions between TRF2TRFH and different MCPH1TBM mutant peptides. S333phos is a phosphorylated S333 peptide synthesized using a phosphorylated serine as starting material. Equilibrium dissociation constant (KD) values derived from ITC data are shown in Table 2.