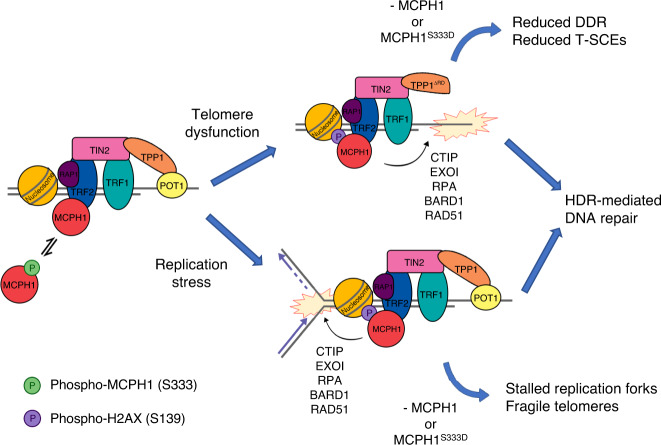
Fig. 7. Schematic depicting MCPH1’s role at telomeres.
MCPH1 interacts with TRF2 when S333 is de-phosphorylated, while this interaction is disrupted upon S333 phosphorylation. In unperturbed conditions, MCPH1 exists in equilibrium between the phosphorylated and the de-phosphorylated forms and its telomeric localization increases in S phase. Removal of POT1 through the overexpression of TPP1ΔRD increases MCPH1 localization to telomeres that is dependent upon the interaction with both TRF2 and γ-H2AX. MCPH1 binding to TRF2 promotes the recruitment of HDR and end resection factors to initiate HDR at telomeres lacking POT1-TPP1. Cells lacking MCPH1 or expressing MCPH1S333D display reduced recruitment of HDR factors and reduced T-SCEs, suggestive of HDR defects. Similarly, induction of replication stress at telomeres increases MCPH1 telomeric localization, promoted by its interaction with TRF2. In the absence of MCPH1 or upon overexpression of the phosphomimetic mutant MCPH1S333D, replication forks stalling at telomeres increases and telomere replication is impaired, resulting in telomere fragility. These observations suggest that telomeric localization of MCPH1 is required for proper telomere replication by promoting stalled replication fork restart.