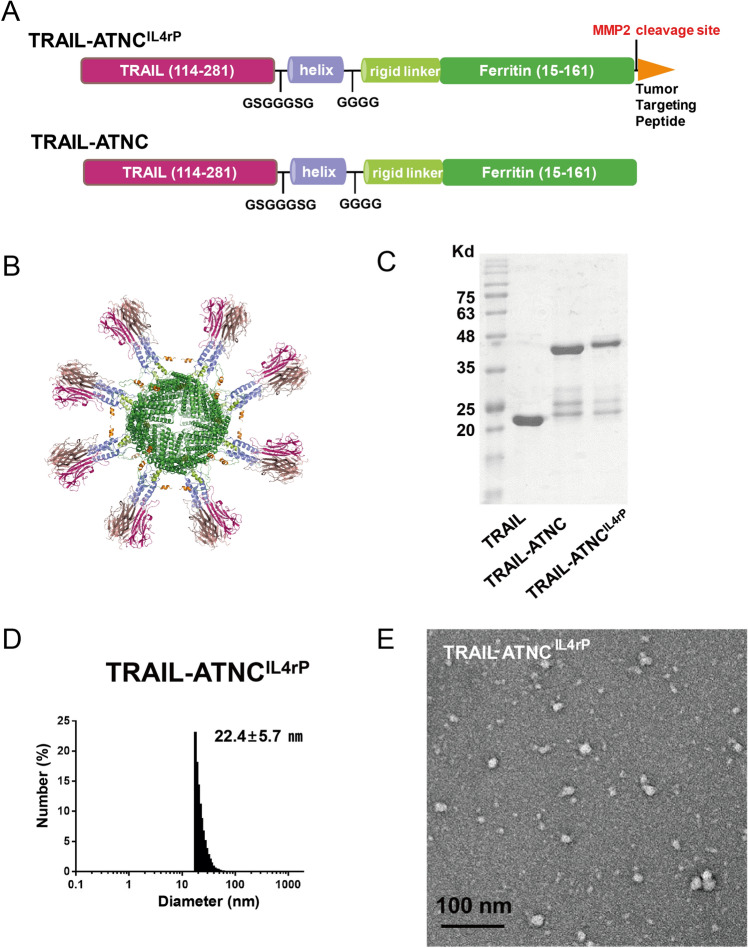
Figure 1.
Design and physicochemical characterization of TRAIL-Active Trimer Nanocage (ATNC). (A) Protein primary structure diagram of TRAIL-ATNC and TRAIL-ATNCIL4rP. The C terminus of the ecto-domain of TRAIL (114–281) was fused to the N-terminus of the short version of the human ferritin subunit (15–161) by a helix and rigid linker. The IL4 receptor binding peptide (IL4rP: CRKRLDRNC) was inserted into the C-terminal of the ferritin as a tumor targeting peptide. A matrix metalloproteinase-2 (MMP2) cleavage site (GPLGLAG) was inserted between the IL4rP and the ferritin. (B) 3D picture of the TRAIL-ATNCIL4rP with eight TRAIL homotrimers displayed on the surface of ferritin nanocages. The image was drawn using PyMOL v0.99 (The PyMOL Molecular Graphics System, Schrödinger, LLC). (C) SDS-PAGE of the purified TRAIL-ATNC and TRAIL-ATNCIL4rP proteins. The major bands correspond with the expected sized of TRAIL-ATNC (44.1 kDa) and TRAIL-ATNCIL4rP (46.7 kDa). (D) DLS analysis of TRAIL-ATNCIL4rP. (E) Transmission electron microscopy image of TRAIL-ATNCIL4rP.