Abstract
Objective
This post hoc analysis evaluated the safety and efficacy of open‐label sarilumab in patients with rheumatoid arthritis (RA) who completed the phase III double‐blind ASCERTAIN study (NCT01768572) and switched from intravenous (IV) tocilizumab to subcutaneous (SC) sarilumab, or who continued SC sarilumab in the open‐label extension (OLE) study EXTEND (NCT01146652).
Methods
Patients who completed ASCERTAIN were eligible to enroll in EXTEND to receive sarilumab 200 mg SC every 2 weeks (Q2W). Safety and efficacy were reported through 96 weeks in the OLE in patients who switched from tocilizumab IV to sarilumab 200 mg SC Q2W, who switched from sarilumab 150 mg SC Q2W to sarilumab 200 mg SC Q2W, or who continued sarilumab 200 mg SC Q2W.
Results
Of 175 patients who completed ASCERTAIN, 168 (96%) enrolled in EXTEND, and 38 of these patients (23%) discontinued the OLE. Cumulative sarilumab exposure during follow‐up was 273.7 patient‐years. No new safety signals were identified, infections occurred at a rate of 59.9/100 patient‐years, and there were no cases of grade 4 neutropenia. Efficacy—as assessed by Disease Activity Score (28 joints) based on C‐reactive protein, Clinical Disease Activity Index, and Health Assessment Questionnaire‐Disability Index scores—was sustained over 96 weeks of follow‐up when switching to, or continuing, sarilumab 200 mg SC Q2W.
Conclusion
Switching from IV to SC interleukin‐6 receptor inhibitor therapy produced no new safety concerns, and clinical efficacy was sustained over 96 weeks of follow‐up. These findings alleviate potential concerns over switching route of administration with interleukin‐6 receptor inhibitor therapy for RA.
INTRODUCTION
Sarilumab is a human monoclonal antibody that binds to both the membrane‐bound and soluble forms of the interleukin‐6 receptor (IL‐6R), and functions to inhibit IL‐6 signaling (1). It is approved for the treatment of moderately to severely active rheumatoid arthritis (RA) in adult patients who have had an inadequate response to, or intolerance of, one or more disease‐modifying antirheumatic drugs (DMARDs), and it can be used either as monotherapy or concomitantly with methotrexate or other conventional synthetic DMARDs (csDMARDs) (1). Sarilumab has demonstrated clinical and radiographic benefits in adult patients with RA in active‐comparator and placebo‐controlled phase III trials, both as a monotherapy and in combination with csDMARDs (2, 3, 4, 5). Tocilizumab is a humanized recombinant monoclonal antibody that targets membrane‐bound and soluble IL‐6R and is approved for the treatment of RA (6).
The phase III ASCERTAIN study (NCT01768572) was a 24‐week, randomized, double‐blind, double‐dummy, three‐arm, parallel‐group safety study in patients with active RA who had an inadequate response to a prior tumor necrosis factor inhibitor and were receiving csDMARD treatment (2). The study demonstrated that sarilumab and tocilizumab were each well tolerated and had similar safety profiles. Sarilumab was administered by subcutaneous (SC) injection, and tocilizumab was administered intravenously (IV) according to its US product labeling at the time the study was conducted (6). After the double‐blind randomized control phase (RCT), patients were eligible to enroll in the ongoing open‐label extension (OLE) study, EXTEND (NCT01146652). EXTEND is a 5‐year extension study to assess the long‐term safety and efficacy of sarilumab SC in patients with RA who previously participated in one of several shorter‐term sarilumab studies (2, 4, 7, 8). Data presented here show only those patients in EXTEND who enrolled from the ASCERTAIN RCT. Patients who received tocilizumab IV plus placebo SC in the RCT switched to sarilumab SC on enrollment into the OLE. This switch made it possible to investigate the effects of switching from IV to SC IL‐6R inhibition in a clinical trial setting. The aim of this post hoc analysis was to evaluate the safety and efficacy of switching from double‐blind, double‐dummy tocilizumab IV or sarilumab SC to open‐label sarilumab SC in the OLE.
PATIENTS AND METHODS
Ethics approval
The protocol was approved by the appropriate ethics committees/institutional review boards: London ‐ Brighton & Sussex Research Ethics Committee (approval number 13/LO/0499) and from each of the 85 additional study centers in Europe (including Russia), North America, and South America. Each patient gave written informed consent before study participation. The study was conducted in compliance with institutional review board regulations, the International Conference on Harmonization Guidelines for Good Clinical Practice, and the Declaration of Helsinki.
Study design
In ASCERTAIN, patients were randomized 1:1:2 to receive sarilumab 150 mg SC every 2 weeks (Q2W), sarilumab 200 mg SC Q2W, or tocilizumab 4 mg/kg IV every 4 weeks (Q4W) (2). In instances of an insufficient response to tocilizumab 4 mg/kg IV Q4W, the dose could be increased to 8 mg/kg IV Q4W at the investigator’s discretion, per US label instructions (6). EXTEND is a multicenter OLE study designed to assess the long‐term safety and efficacy of sarilumab SC in adult patients with RA, including in patients who completed the ASCERTAIN study. All patients who completed the parent study were eligible for inclusion in the OLE. Patients entering the OLE received sarilumab 200 mg SC Q2W.
Assessments
OLE baseline was defined as the start of the OLE (visit 1, week 0). Safety data were reported from patient visit 1 to visit 14 (OLE week 96) for all patients who entered the OLE. Visits occurred Q2W until OLE week 12 and every 12 weeks thereafter until OLE week 96. Assessments included the recording of treatment‐emergent adverse events (AEs) and laboratory investigations, including absolute neutrophil counts and liver function tests. Samples for laboratory investigations were taken at each visit. AEs were described using the Medical Dictionary for Regulatory Activities (version 20.0) preferred terms. AEs of special interest were collected according to predefined criteria, specific to scientific and medical concerns of the drugs involved.
Efficacy assessments—using the Disease Activity Score (28 joints) based on C‐reactive protein (DAS28‐CRP), Clinical Disease Activity Index (CDAI), and Health Assessment Questionnaire‐Disability Index (HAQ‐DI)—were made at each study visit. Continuous efficacy outcomes are presented as observed cases (OCs) without imputation of missing data as mean score (±SE). Disease activity thresholds were DAS28‐CRP < 2.6 or < 3.2, CDAI ≤ 2.8 or ≤ 10, and HAQ‐DI score improvement of ≥0.3 from score at RCT baseline. The proportions of patients achieving DAS28‐CRP, CDAI, or HAQ‐DI disease activity thresholds at weeks 12 and 96 are presented in two ways: (a) based on the number of patients assessed at each visit (OC approach) or (b) based on the intention‐to‐treat (ITT) population approach, with patients for whom data were missing counted as nonresponders (nonresponder imputation).
Data in the OLE were compared according to patients’ original treatment groups in the RCT: sarilumab 150 mg SC Q2W, sarilumab 200 mg SC Q2W, or tocilizumab 4 mg/kg IV Q4W received throughout the RCT, or tocilizumab 4 mg/kg IV Q4W increased to 8 mg/kg IV Q4W at week 4 of the RCT and continuing at 8 mg/kg IV Q4W thereafter (referred to as the tocilizumab 4/8 mg/kg group). Data from the OLE were also grouped into all sarilumab patients and all tocilizumab patients. To maintain consistency within the groups, patients who changed tocilizumab dose after week 4 were not included in the tocilizumab 4 mg/kg group or the 4/8 mg/kg group, but only in the all‐tocilizumab group. Data were analyzed using descriptive statistics only.
Patient and public involvement
The clinical trial was recorded on public registry websites prior to the enrollment of the first patient. This research was done without patient consultation. At the time that this study was conducted, there were no funds or time allocated for patient/public involvement in study design or result‐dissemination planning.
Open access
This is an open access article distributed in accordance with the Creative Commons Attribution‐NonCommercial (CC BY‐NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work noncommercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is noncommercial. See: http://creativecommons.org/licenses/by‐nc/4.0/.
RESULTS
Patient population
Of the 175 patients who completed the RCT, 168 (96%) enrolled in the OLE. A similar number of patients enrolled from each RCT treatment group: sarilumab 150 mg (n = 37, 22% of patients in the OLE), sarilumab 200 mg (n = 38, 23%), tocilizumab 4 mg/kg (n = 35, 21%), and tocilizumab 4/8 mg/kg (n = 38, 23%). An additional 20 patients who changed tocilizumab dosage after week 4 of the parent study were included in the all‐tocilizumab group, so the tocilizumab 4/8 mg/kg group was consistent in containing only patients who changed dose from 4 to 8 mg/kg IV Q4W at week 4 and remained on tocilizumab 8 mg/kg IV Q4W thereafter. Patient demographic and most disease characteristics were well balanced across all groups, although, compared with other treatment groups, baseline CRP levels were elevated in patients who had received only tocilizumab 4 mg/kg for 24 weeks and were not escalated to 8 mg/kg during the RCT (Table 1).
Table 1.
Demographics and baseline disease characteristics
| Treatment During the RCT, Before Switch to Sarilumab 200 mg SC Q2W in the OLE | |||||
|---|---|---|---|---|---|
|
Sarilumab 150 mg SC Q2W (n = 37) |
Sarilumab 200 mg SC Q2W (n = 38) |
Tocilizumab 4 mg/kg IV Q4W a (n = 35) |
Tocilizumab 4/8 mg/kg IV Q4W b (n = 38) |
All tocilizumab c (n = 93) | |
| Recorded at RCT baseline | |||||
| Female, n (%) | 30 (81.1) | 31 (81.6) | 29 (82.9) | 31 (81.6) | 76 (81.7) |
| Duration of RA since diagnosis (y), mean (SD) | 12.9 (7.3) | 8.8 (6.0) | 9.1 (7.3) | 11.5 (7.8) | 9.9 (7.9) |
| ACR RA functional class, n (%) | |||||
| I | 6 (16.2) | 3 (7.9) | 6 (17.1) | 3 (7.9) | 13 (14.0) |
| II | 20 (54.1) | 24 (63.2) | 24 (68.6) | 21 (55.3) | 58 (62.4) |
| III | 11 (29.7) | 11 (28.9) | 5 (14.3) | 14 (36.8) | 22 (23.7) |
| IV | 0 | 0 | 0 | 0 | 0 |
| RF positive, n (%) | 29 (80.6) d | 23 (60.5) | 28 (80.0) | 30 (81.1) e | 73 (79.3) f |
| ACPA positive, n (%) | 32 (88.9) d | 30 (78.9) | 28 (80.0) | 31 (91.2) g | 76 (86.4) h |
| Recorded at OLE baseline, mean (SD) | |||||
| Age, y | 53.5 (10.8) | 52.2 (11.5) | 51.2 (13.3) | 50.1 (12.0) | 50.4 (12.8) |
| BMI, kg/m2 | 27.1 (5.1) | 29.3 (6.8) | 29.2 (4.3) | 27.4 (6.1) | 28.0 (5.3) |
| Nonbiologic DMARD use, n (%) | 37 (100) | 37 (97.4) | 35 (100) | 37 (97.4) | 92 (92.9) |
| Glucocorticoid use, n (%) | 16 (43.2) | 15 (39.5) | 19 (54.3) | 23 (60.5) | 49 (52.7) |
| Disease activity at OLE baseline | |||||
| Tender joint count (0–68) | 7.1 (8.9) | 7.2 (8.7) | 4.5 (4.8) | 7.5 (7.9) | 6.3 (7.2) |
| Swollen joint count (0–66) | 3.1 (3.5) | 4.5 (6.4) | 3.3 (3.4) | 3.9 (3.8) | 4.0 (4.8) |
| CRP (mg/L) | 5.4 (13.8) d | 2.2 (5.3) | 14.1 (16.4) g | 3.1 (8.4) | 6.7 (12.6) f |
| DAS28‐CRP | 3.0 (1.2) d | 3.0 (1.2) | 3.3 (1.2) g | 3.2 (1.1) | 3.2 (1.2) f |
| CDAI | 11.9 (10.3) | 13.0 (11.8) | 10.6 (7.5) | 13.7 (9.0) | 12.4 (9.5) |
| HAQ‐DI (0–3) | 1.1 (0.6) | 1.2 (0.6) | 1.0 (0.8) | 1.3 (0.7) | 1.2 (0.7) |
Abbreviations: ACPA, anticyclic citrullinated peptide antibody; ACR, American College of Rheumatology; BMI, body mass index; CDAI, Clinical Disease Activity Index; CRP, C‐reactive protein; DAS28‐CRP, Disease Activity Score (28 joints) based on CRP; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; IV, intravenous; OLE, open‐label extension; Q2W, every 2 weeks; Q4W, every 4 weeks; RA, rheumatoid arthritis; RCT, double‐blind randomized control phase; RF, rheumatoid factor; SC, subcutaneous; SD, standard deviation.
Patients received tocilizumab 4 mg/kg IV Q4W at baseline in the RCT and remained at this dose throughout.
Patients received tocilizumab 4 mg/kg IV Q4W at baseline in the RCT and switched at week 4 to tocilizumab 8 mg/kg IV Q4W for the remainder of the study.
Included n = 20 patients who changed tocilizumab dose after week 4 of the RCT.
n = 36.
n = 7.
n = 92.
n = 34.
n = 88.
Safety
Overall, 38 patients (23%) discontinued the OLE study before week 96. Discontinuations due to AEs were reported for 22 patients (13%). Discontinuations for reasons other than safety included 5 patients (3%) due to lack of efficacy and 1 patient (1%) due to poor compliance with the protocol; 14 patients (8%) requested treatment discontinuation (Figure 1). Overall discontinuation rates were similar across the prior treatment groups in the RCT, except in the tocilizumab 4/8 mg/kg group, which had a greater number of discontinuations, mainly due to patient‐requested discontinuations (n = 7, 18%). Three deaths of patients treated with open‐label sarilumab were recorded during the OLE: one in the original sarilumab 150 mg RCT group (cervical cancer), one in the original sarilumab 200 mg RCT group (colorectal cancer), and one in the original tocilizumab 4 mg/kg RCT group (psoas abscess; Table 2).
Figure 1.

Patient disposition through the OLE. Abbreviations: IV, intravenous; OLE, open‐label extension; Q2W, every 2 weeks; Q4W, every 4 weeks; RCT, double‐blind randomized control phase; SC, subcutaneous.
aPatients requesting discontinuation is not additive with the number of patients who discontinued.
Table 2.
Investigator‐reported AEs
| Treatment During the RCT, Before Switch to Sarilumab 200 mg SC Q2W in the OLE | ||||||
|---|---|---|---|---|---|---|
|
All patients (n = 168) |
Sarilumab 150 mg SC Q2W (n = 37) |
Sarilumab 200 mg SC Q2W (n = 38) |
Tocilizumab 4 mg/kg IV Q4W a (n = 35) |
Tocilizumab 4/8 mg/kg IV Q4W b (n = 38) |
All tocilizumab c (n = 93) |
|
| Cumulative total AE observation period, PY | 273.7 | 61.2 | 63.6 | 56.7 | 57.4 | 149.0 |
| Summary, d n (%) [nE/100 PY] | ||||||
| Any AE | 137 (81.5) [276.2] | 31 (83.8) [376.0] | 30 (78.9) [291.0] | 25 (71.4) [195.6] | 33 (86.8) [250.8] | 76 (81.7) [228.9] |
| SAE | 32 (19.0) [15.7] | 11 (29.7) [22.9] | 5 (13.2) [9.4] | 7 (20.0) [19.4] | 7 (18.4) [13.9] | 16 (17.2) [15.4] |
| AE leading to permanent discontinuation e | 21 (12.5) [7.7] | 4 (10.8) [6.5] | 5 (13.2) [7.9] | 5 (14.3) [8.8] | 6 (15.8) [10.5] | 12 (12.9) [8.1] |
| AE leading to death | 3 (1.8) [1.1] | 1 (2.7) [1.6] | 1 (2.6) [1.6] | 1 (2.9) [1.8] | … | 1 (1.1) [0.7] |
| AEs with incidence ≥ 10% in any group, f n (%) [nE/100 PY] | ||||||
| Neutropenia | 24 (14.3) [19.4] | 3 (8.1) [9.8] | 5 (13.2) [12.6] | 5 (14.3) [28.2] | 7 (18.4) [15.7] | 16 (17.2) [26.2] |
| Upper respiratory tract infection | 20 (11.9) [8.8] | 6 (16.2) [14.7] | 4 (10.5) [6.3] | 5 (14.3) [10.6] | 4 (10.5) [7.0] | 10 (10.8) [7.4] |
| Urinary tract infection | 15 (8.9) [6.6] | 5 (13.5) [11.4] | 2 (5.3) [3.1] | 3 (8.6) [5.3] | 5 (13.2) [10.5] | 8 (8.6) [6.0] |
| Injection‐site erythema | 15 (8.9) [30.3] | 4 (10.8) [44.1] | 5 (13.2) [59.8] | 2 (5.7) [14.1] | 3 (7.9) [15.7] | 6 (6.5) [12.1] |
| Rheumatoid arthritis | 15 (8.9) [6.9] | 3 (8.1) [4.9] | 4 (10.5) [9.4] | 2 (5.7) [3.5] | 3 (7.9) [8.7] | 8 (8.6) [6.7] |
| Viral upper respiratory tract infection | 14 (8.3) [6.2] | 4 (10.8) [9.8] | 3 (7.9) [4.7] | 1 (2.9) [1.8] | 3 (7.9) [5.2] | 7 (7.5) [5.4] |
| Back pain | 11 (6.5) [4.4] | 4 (10.8) [6.5] | 2 (5.3) [4.7] | … | 4 (10.5) [7.0] | 5 (5.4) [3.4] |
| Diarrhea | 9 (5.4) [3.3] | 2 (5.4) [3.3] | 1 (2.6) [1.6] | 1 (2.9) [1.8] | 4 (10.5) [7.0] | 6 (6.5) [4.0] |
| Fall | 6 (3.6) [2.2] | 5 (13.5) [8.2] | … | … | 1 (2.6) [1.7] | 1 (1.1) [0.7] |
| Headache | 6 (3.6) [2.2] | 4 (10.8) [6.5] | … | … | 2 (5.3) [3.5] | 2 (2.2) [1.3] |
Abbreviations: AE, treatment‐emergent adverse event; IV, intravenous; nE/100 PY, number of events per 100 PY; OLE, open‐label extension; PY, patient‐years; Q2W, every 2 weeks; Q4W, every 4 weeks; RCT, double‐blind randomized control phase; SAE, serious adverse event; SC, subcutaneous.
Patients received tocilizumab 4 mg/kg IV Q4W at baseline in the RCT and remained at this dose throughout.
Patients received tocilizumab 4 mg/kg IV Q4W at baseline in the RCT and switched at week 4 to tocilizumab 8 mg/kg IV Q4W for the remainder of the study.
Included n = 20 patients who changed tocilizumab dose after week 4 of the RCT.
Incidence rate (ne/100 PY) for summary is during time to first event.
Excluding one patient (sarilumab + disease‐modifying antirheumatic drug group) with pretreatment AEs leading to permanent treatment discontinuation.
Incidence rate is over cumulative total AE observation period.
The overall incidence and exposure‐adjusted rates of investigator‐reported AEs during the OLE were similar among groups (Table 2). No new safety signals emerged in patients who switched from tocilizumab IV to sarilumab SC, nor during longer‐term treatment in those continuing sarilumab. The most common AEs (greatest percentages of patients with at least one event) were neutropenia (19.4/100 patient‐years [PY] overall), upper respiratory tract infections (8.8/100 PY), and urinary tract infections (6.6/100 PY). The most common AEs of special interest were infections (59.9/100 PY overall), injection‐site reactions (42.4/100 PY), and leukopenia (23.7/100 PY; Supplementary Table 1). Serious infections occurred at a rate of 3.3/100 PY. The incidence of leukopenia was similar across groups. There was one upper gastrointestinal (GI) perforation in the OLE (RCT tocilizumab 4 mg/kg group) but no lower GI perforations. Malignancies occurred in five patients (1.8/100 PY), three of which were nonmelanoma skin cancer, one was cervical cancer, and one was colorectal cancer.
Incidence of investigator‐reported neutropenia was generally similar across groups (Table 2). Protocolled laboratory investigations detected absolute neutrophil count (ANC) nadir of ≥500 to 1000 cells/mm3 (grade 3 neutropenia) in 17 patients overall (10.1%; Supplementary Table 2). No patients had ANC < 500 cells/mm3 (grade 4 neutropenia). Liver function test abnormalities were similar across groups, with 10 patients (6.0%) exhibiting alanine aminotransferase (ALT) > 3 × the upper limit of normal and 2 patients (1.2%) exhibiting ALT > 5 × the upper limit of normal (Supplementary Table 3). There were no cases of Hy’s law (drug‐induced liver injury). Investigator‐reported lipid elevations occurred with an incidence rate of 6.9/100 PY (Supplementary Table 1).
Efficacy
Mean DAS28‐CRP, CDAI, and HAQ‐DI scores were generally sustained over 96 weeks of follow‐up in patients who switched from tocilizumab 4 or 4/8 mg/kg IV Q4W, or who remained on sarilumab SC (Figure 2). In patients with low disease activity (DAS28‐CRP < 3.2 or CDAI ≤ 10) at entry into the OLE, low disease activity thresholds were largely maintained through week 96 of the OLE (Figure 3). A small number of patients who did not achieve low disease activity at the end of the RCT achieved low disease activity during the OLE (Supplementary Table 4). Similar results were observed for other disease activity thresholds (Supplementary Figure 1; Supplementary Table 4).
Figure 2.
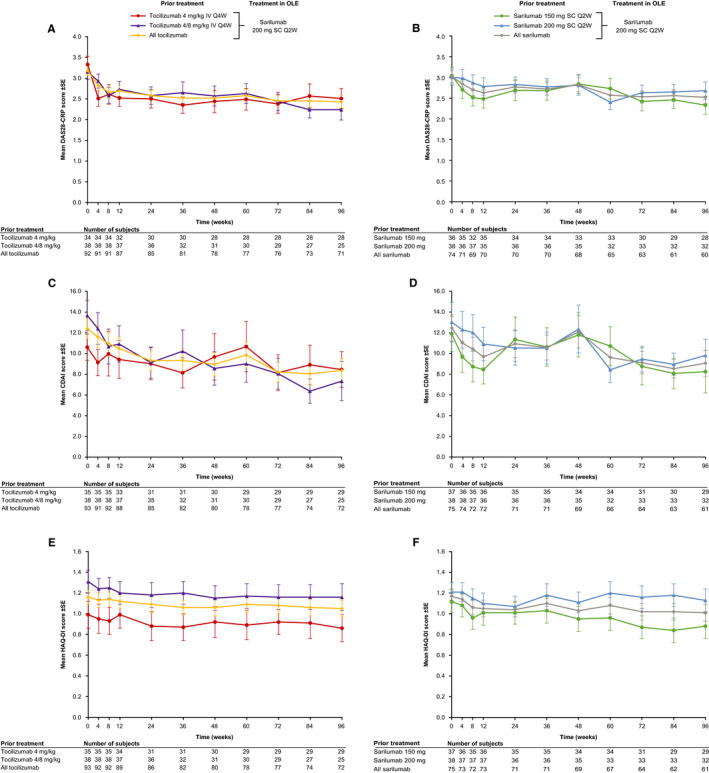
Mean (±SE) efficacy scores from OLE baseline to week 96 in overall patient population (observed cases) for DAS28‐CRP (A, B), CDAI (C, D), and HAQ‐DI (E, F). Abbreviations: CDAI, Clinical Disease Activity Index; DAS28‐CRP, Disease Activity Score (28 joints) based on C‐reactive protein; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; IV, intravenous; OLE, open‐label extension; Q2W, every 2 weeks; Q4W, every 4 weeks; SC, subcutaneous.
Figure 3.

Percentage of patients (OC and ITT) who achieved disease activity thresholds at OLE baseline and sustained them following switch to open‐label sarilumab at OLE weeks 12 and 96.
Abbreviations: CDAI, Clinical Disease Activity Index; DAS28‐CRP, Disease Activity Score (28 joints) based on C‐reactive protein; ITT, intention‐to‐treat patient population; IV, intravenous; OC, observed case patient population; OLE, open‐label extension; Q2W, every 2 weeks; Q4W, every 4 weeks; SC, subcutaneous.
aUses the number of patients assessed at each time point as the denominator for the percentage of patients achieving disease activity thresholds at each time point.
bUses the number of patients achieving disease activity thresholds at OLE baseline as the denominator for the percentage of patients achieving disease activity thresholds at each time point.
DISCUSSION
In this study, patients who switched from IV to SC administration of IL‐6R inhibitors for treatment of RA experienced sustained tolerability and efficacy. No new safety signals emerged during the OLE in patients who switched from tocilizumab IV to sarilumab SC, nor during longer‐term treatment in those continuing sarilumab. Clinical efficacy was generally sustained after switching to sarilumab SC across the whole patient cohort through 96 weeks of follow‐up, regardless of initial treatment in the RCT.
There is a trend for increasing use of SC over IV administration in RA (9, 10), and evidence that both physicians and patients prefer SC therapy over IV (11, 12, 13). The convenience of home treatment, faster treatment time, lower costs, reduced travel time, and fewer visits to clinics or hospitals were all cited as advantages of SC treatment versus IV, though some patients preferred IV treatment because it meant administration by a medical professional and interaction with other patients during treatment (11, 12, 14, 15, 16, 17, 18, 19). There is also an association between requirements for longer‐term treatment and patient preference for SC therapy (13), which is an important consideration for the management of a chronic condition such as RA. In this and other studies of patients with RA, patients who switched from IV to SC therapy generally maintained SC treatment after switching (20, 21), which suggests that SC treatment was as satisfactory as IV. The results presented here offer reassurance to physicians and patients considering a switch in route of administration for IL‐6R inhibitor therapy for RA and are consistent with evidence of continued efficacy and safety following IV to SC switch with other RA therapies (10, 20, 22, 23).
The safety profile in patients who switched to open‐label sarilumab 200 mg Q2W was generally consistent with that observed for sarilumab 200 mg Q2W treatment during the RCT (2), with previous analyses of phase III studies of sarilumab (4, 5, 7, 24), and with the safety profile expected of IL‐6 inhibition (25). Incidences of elevated levels of ALT and other markers of liver function were comparable across all groups and consistent with those in previous studies (2, 4). The small number of patients who reported injection‐site reactions according to the composite AESI term (n = 4‐5 in each group) does not support firm conclusions. However, the uniformity of the administration method across groups (“double‐dummy, double‐blind” SC and IV injections before switch; open‐label sarilumab after switch) might explain the relatively uniform proportion of patients reporting injection‐site reactions after switch.
The observation that baseline CRP levels were higher after tocilizumab 4 mg/kg IV Q4W compared with patients who received tocilizumab 8 mg/kg IV Q4W, or sarilumab 150 or 200 mg SC (despite similar CRP levels at the start of the randomization) (2), is consistent with other studies that have shown dose‐dependent and potential suboptimal suppression of CRP with the lower tocilizumab dose (26).
Efficacy was generally sustained in patients who continued from sarilumab in the RCT to open‐label sarilumab in the OLE. There have been concerns that unblinding patients following commencement of open‐label treatment may introduce bias due to altered perceptions in patient self‐reporting or changes in placebo effects (27). These issues do not seem to have had an effect on the outcomes in the present study, in which clinical assessment scores remained stable in the sarilumab continuation groups after switching to open‐label sarilumab. The proportion of patients who achieved disease activity thresholds (DAS28‐CRP < 2.6 or < 3.2, CDAI ≤ 2.8 or ≤ 10, or HAQ‐DI score improvement ≥ 0.3) at the end of the RCT was also generally stable through 96 weeks of follow‐up in the OLE study after switching to open‐label sarilumab 200 mg SC Q2W, regardless of initial treatment in the RCT.
A subset of patients who did not achieve disease activity thresholds at the end of the RCT subsequently achieved them following a switch to open‐label sarilumab. These improvements in disease activity postswitch may be attributed to a number of reasons, including late response to anti–IL‐6R treatment, positive effects resulting from the change to an open‐label treatment in the OLE, or unknown mechanistic differences between the two therapies.
Limitations of this study include that it is a post hoc evaluation of a relatively small subpopulation of patients from the EXTEND OLE trial, with no formal statistical analysis. The switch to sarilumab was performed in an OLE, which has the potential to introduce a positive bias into the treatment population. This can occur because patients become aware that they are receiving the optimal dose of the experimental treatment option. Bias can also occur because the OLE discounts noncompleters in the RCT section of the study, which can enrich the study population with patients who tolerate and respond well to treatment. This can lead to a progressively smaller pool of patients, most of whom have a positive treatment response. To overcome this bias, we reported proportions of patients using ITT analysis as well as OC analysis.
In conclusion, switching from double‐blind tocilizumab IV or sarilumab SC to open‐label sarilumab SC produced no new safety concerns and demonstrated sustained clinical efficacy over 96 weeks, as shown by the durability of treatment effect. The safety profile of sarilumab in the OLE was generally consistent with that seen in the RCT, with long‐term sarilumab treatment, and with the anticipated profile of an IL‐6 inhibitor.
AUTHOR CONTRIBUTIONS
All authors contributed to the design of this analysis or to the analysis or interpretation of the data. PE and PV contributed to data acquisition. All authors contributed to manuscript development, approved the final version, and agree to be accountable for all aspects of the work. Qunming Dong, formerly an employee of Sanofi, contributed to the initial design of this analysis and to data analysis/interpretation. No other disclosures relevant to this article were reported.
Supporting information
Supplementary Material
Acknowledgments
We thank Qunming Dong (a Sanofi employee at the time of the study) and Tejasweeni Rajput (Cytel) for their statistical support. Medical writing support was provided by Joseph Hodgson, PhD (Adelphi Communications Ltd, Macclesfield, UK) and was funded by Sanofi Genzyme (Cambridge, Massachusetts) and Regeneron Pharmaceuticals, Inc. (Tarrytown, New York) in accordance with Good Publication Practice guidelines. Data in this manuscript expand on those previously presented at the 2018 European League Against Rheumatism Annual Meeting: Verschueren P, Emery P, van Hoogstraten H, Dong Q, Mangan EK, den Broeder A. Efficacy of sarilumab in patients with rheumatoid arthritis with and without previous response to tocilizumab. Proceedings of European League Against Rheumatism Annual Meeting, 2018, June 13‐16, Amsterdam; Ann Rheum Dis 2018;77 Suppl 2:327.
This study was supported by Sanofi Genzyme (Cambridge, Massachusetts) and Regeneron Pharmaceuticals, Inc. (Tarrytown, New York), which developed sarilumab.
Prof. Emery has received grant/research support from, and provided consultancy for, AbbVie, Bristol‐Myers Squibb, Merck Sharp & Dohme, Pfizer, and Roche; has provided consultancy for Novartis and UCB; and has received personal fees from Lilly, Samsung, and Sandoz. Dr. van Hoogstraten is an employee of Sanofi, and may hold stock and/or stock options in the company. Dr. Thangavelu is a former employee of Sanofi Genzyme, is currently employed by EMD Serono, and may hold stock and/or stock options in these companies. Dr. Mangan is a former employee of Regeneron Pharmaceuticals, Inc., and may hold stock and/or stock options in the company. Dr. St John is a former employee of Regeneron Pharmaceuticals, Inc., is currently employed by Intercept Pharmaceuticals, Inc., and may hold stock and/or stock options in these companies. Prof. Verschueren has received consulting fees from AbbVie, Bristol‐Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Nordic Pharma, Pfizer, Roche, Sanofi, and UCB, and has been a paid instructor for Pfizer and Sanofi.
REFERENCES
- 1. Kevzara (sarilumab) injection, for subcutaneous use. Initial US approval. Silver Spring (MD): U.S. Food and Drug Administration; 2017. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf.
- 2. Emery P, Rondon J, Parrino J, Lin Y, Pena‐Rossi C, van Hoogstraten H, et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford) 2019;58:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burmester GR, Lin Y, Mangan EK, van Hoogstraten H, Kimura T, Vargas JI, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy in patients with active rheumatoid arthritis in the phase 3 monarch study, including subpopulations. Ann Rheum Dis 2017;76:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleischmann R, van Adelsberg J, Lin Y, Castelar‐Pinheiro GD, Brzezicki J, Hrycaj P, et al. Sarilumab and nonbiologic disease‐modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genovese MC, Fleischmann R, Kivitz AJ, Rell‐Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 6. Actemra (tocilizumab) injection for intravenous use injection, for subcutaneous use [package insert]. Silver Spring (MD): U.S. Food and Drug Administration; 2017. URL: www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf.
- 7. Burmester GR, Lin Y, Patel R, van Adelsberg J, Mangan EK, Graham NM, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double‐blind, parallel‐group phase III trial. Ann Rheum Dis 2017;76:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells AF, Parrino J, Mangan EK, Paccaly A, Lin Y, Xu C, et al. Immunogenicity of sarilumab monotherapy in patients with rheumatoid arthritis who were inadequate responders or intolerant to disease‐modifying antirheumatic drugs. Rheumatol Ther 2019;6:339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decision Resources Group . Rheumatoid arthritis: disease landscape & forecast (landscape and forecast series). 2017. URL: https://decisionresourcesgroup.com/report/dlsfim0007‐2017‐biopharma‐rheumatoid‐arthritis‐disease‐landscape‐and/.
- 10. Lauper K, Mongin D, Iannone F, Klami Kristianslund E, Kvien TK, Nordström D, et al. Comparative effectiveness of subcutaneous tocilizumab versus intravenous tocilizumab in a pan‐European collaboration of registries. RMD Open 2018;4:e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pivot X, Gligorov J, Muller V, Curigliano G, Knoop A, Verma S, et al. Patients' preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of her2‐positive early breast cancer: final analysis of 488 patients in the international, randomized, two‐cohort PrefHer study. Ann Oncol 2014;25:1979–87. [DOI] [PubMed] [Google Scholar]
- 12. Huynh TK, Ostergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Pref Adherence 2014;8:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoner KL, Harder H, Fallowfield LJ, Jenkins VA. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient 2015;8:145–53. [DOI] [PubMed] [Google Scholar]
- 14. Monti S, Breda S, Grosso V, Todoerti M, Montecucco C, Caporali R. Switching from intravenous to subcutaneous formulation of abatacept: different results in a series of 21 patients. J Rheumatol 2015;42:1993–4. [DOI] [PubMed] [Google Scholar]
- 15. Barton JL. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence 2009;3:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alten R, Krüger K, Rellecke J, Schiffner‐Rohe J, Behmer O, Schiffhorst G, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete‐choice approach. Patient Prefer Adherence 2016;10:2217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin JF, Zhu LL, Chen M, Xu HM, Wang HF, Feng XQ, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence 2015;9:923–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards CJ, Williams EL. Patient preferences in choosing anti‐TNF therapies‐R1. Rheumatology (Oxford) 2006;45:1575–6. [DOI] [PubMed] [Google Scholar]
- 19. Desplats M, Pascart T, Jelin G, Norberciak L, Philippe P, Houvenagel E, et al. Are abatacept and tocilizumab intravenous users willing to switch for the subcutaneous route of administration? A questionnaire‐based study. Clin Rheumatol 2017;36:1395–400. [DOI] [PubMed] [Google Scholar]
- 20. Keystone EC, Kremer JM, Russell A, Box J, Abud‐Mendoza C, Elizondo MG, et al. Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb attune study. Ann Rheum Dis 2012;71:857–61. [DOI] [PubMed] [Google Scholar]
- 21. Darloy J, Segaud N, Salmon JH, Eschard JP, Goëb V, Deprez X, et al. Tocilizumab effectiveness after switching from intravenous to subcutaneous route in patients with rheumatoid arthritis: the RoSwitch study. Rheumatol Ther 2019;6:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogata A, Atsumi T, Fukuda T, Hirabayashi Y, Inaba M, Ishiguro N, et al. Sustainable efficacy of switching from intravenous to subcutaneous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015;67:1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mueller RB, Gengenbacher M, Richter S, Dudler J, Möller B, von Kempis J. Change from subcutaneous to intravenous abatacept and back in patients with rheumatoid arthritis as simulation of a vacation: a prospective phase IV, open‐label trial (A‐BREAK). Arthritis Res Ther 2016;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleischmann R, Genovese MC, Lin Y, St John G, van der Heijde D, Wang S, et al. Long‐term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years’ follow‐up. Rheumatology (Oxford) 2020;59:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, et al. Il‐6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015;38:575–84. [DOI] [PubMed] [Google Scholar]
- 26. Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double‐blind randomized controlled clinical trial of the interleukin‐6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. [DOI] [PubMed] [Google Scholar]
- 27. Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


