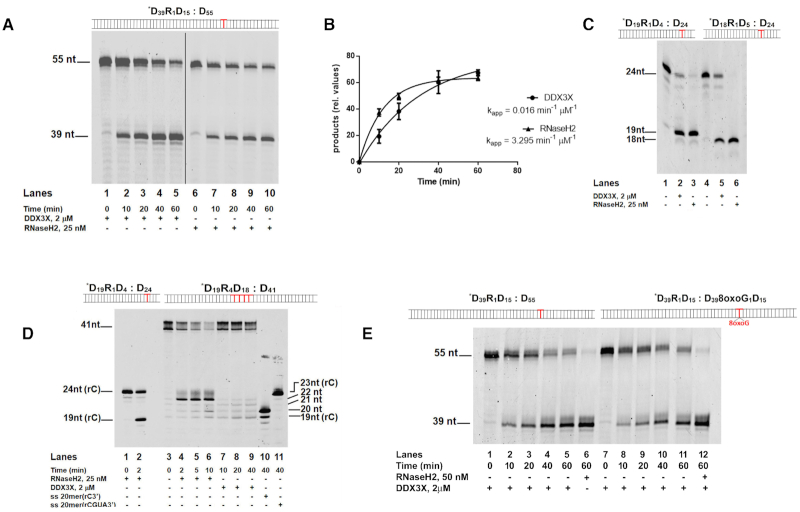
Figure 1.
Human DDX3X has RNaseH2-like activity. (A) Time course of DDX3X (lanes 1–5) and RNaseH2 (lanes 6–10) digestions of Substrate *D39R1D15: D55. (B) Apparent digestion rates for DDX3X and RNaseH2 enzymes on Substrate *D39R1D15: D55. Values are the means of three independent estimates ± S.D. (C) Digestion by DDX3X (lanes 2 and 5) and RNaseH2 (lanes 3 and 6) on Substrate *D19R1D4: D24 and Substrate *D18R1D5: D24 respectively. Lanes 1 and 4: Substrates *D19R1D4: D24 and *D18R1D5: D24 alone, respectively. (D) Substrate *D19R4D18: D41 in the presence of RNaseH2 (lanes 4–6) or DDX3X (lanes 7–9). Lanes 1–2 control reactions with RNaseH2 and Substrate *D19R1D4: D24. Lane 3 Substrate *D19R4D18: D41 alone. Lanes 10, 11 oligonucleotide size markers of defined lengths, used to better identify the different digestion products. (E) Time course of DDX3X digestion of Substrate *D39R1D15: D55 (lanes 1–5) and Substrate *D39R1D15: D398oxoG1D15(lanes 7–11). Control reactions with RNaseH2 of Substrate *D39R1D15: D55 (lane 6) and Substrate *D39R1D15: D398oxoG1D15 (lane 12) respectively.