Figure 1.
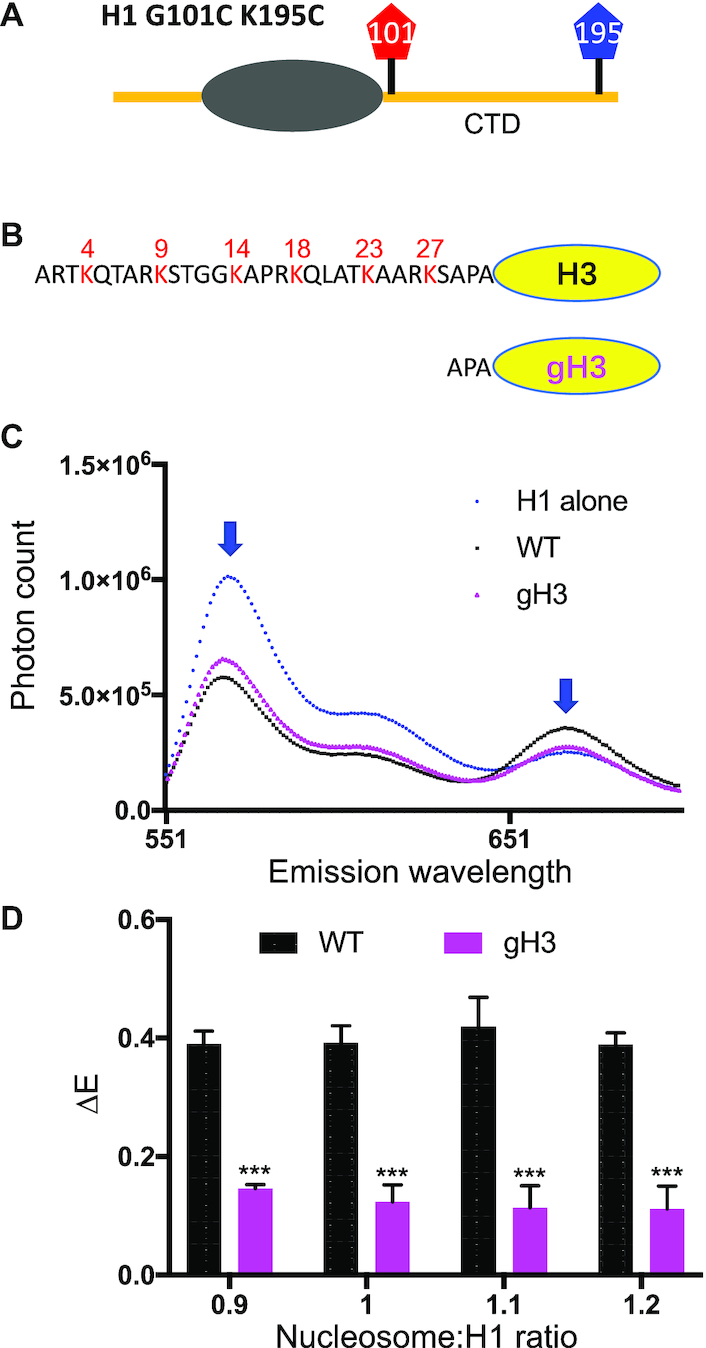
The H3 tail domain influences H1 CTD structure in the nucleosome. Nucleosomes were reconstituted with either full-length H3 (WT) or H3 lacking the N-terminal tail domain (gH3) and FRET assays performed with H1 labeled with Cy3/Cy5 at either end of the H1 CTD. (A) H1 G1010C K195C was labeled with the fluorophores Cy3 and Cy5. (B) Schematic showing WT H3 and deletion of the N-terminal 28 residues in gH3. (C) Emission spectra for H1 alone (blue), and H1 bound to WT nucleosomes (black) or gH3 nucleosomes (magenta). Cy3 excitation was at 515 nm; the Cy3 and Cy5 emission peaks (∼560 and ∼670 nm) are indicated by blue arrowheads. (D) Plot of FRET efficiency difference (ΔE) for samples with the indicated nucleosome:H1 ratios. ΔE was calculated as the difference in FRET efficiency for H1-nucleosome complexes and free H1 for independent trials. Error bars reported are standard deviations (SDs). P values represent probabilities associated with two-tailed Student's t-test. N ≥ 3. (***), P < 0.001.
