Figure 1.
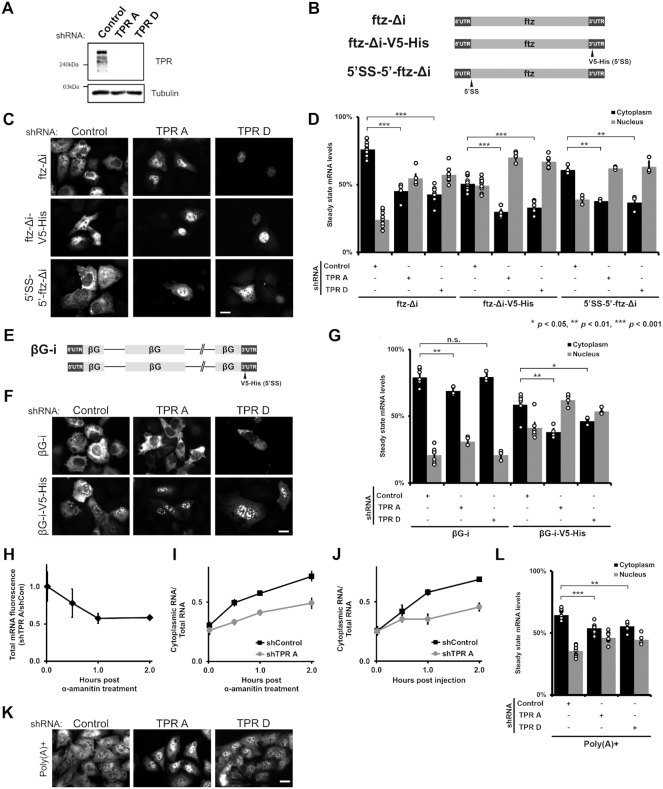
TPR is required for the cytoplasmic accumulation of certain reporter mRNAs but not the nuclear retention of 5′SS motif containing mRNAs. (A) U2OS cells were treated with two different lentiviral delivered shRNAs against TPR (‘TPR A’ and ‘TPR D’) or scrambled control. Lysates were collected after 96 h, separated by SDS-PAGE and immunoprobed for TPR or tubulin. (B) Schematic of the intronless ftz reporter construct used in this study, with and without the V5-His element in the 3′ UTR. The V5-His element contains the 5′SS motif which promotes nuclear retention. For 5′SS-5′-ftz-Δi, the 5′SS motif was inserted into the 5′ end of the ftz reporter. (C, D) Control- or TPR-depleted cells were transfected with the intronless ftz reporter plasmid (±V5-His). 18–24 h later the cells were fixed and the mRNA was visualized by FISH. TPR depletion caused nuclear accumulation of the ftz-Δi mRNA, irrespective of the 5′SS motif. Representative images are shown in (C), scale bar = 10 μm, and quantification is shown in (D) with each bar representing the average and standard error of at least three independent experiments, each experiment consisting of at least 30 to 60 cells. Student t-test was performed for Figure 1D, G and L, * P < 0.05, ** P < 0.01, *** P < 0.001. (E) Schematic of the intron containing βG-i reporter mRNA, with and without the V5-His element. (F, G) Similar to (C, D), except that βG-i reporter plasmid was used. TPR depletion did not significantly affect the mRNA distribution of the βG-i reporter mRNA but increased the nuclear accumulation of βG-i-V5-His mRNA. Scale bar = 10 μm. Representative images are shown in (F), and quantification is shown in (G) with each bar representing the average and standard error of at least three independent experiments, each experiment consisting of at least 30 to 60 cells. (H, I) ftz-Δi plasmid was microinjected into the nuclei of Control- or TPR-depleted U2OS cells. After allowing mRNA synthesis for 20 min, cells were treated with α-amanitin and then incubated for various times to allowed for mRNA export. Cells were fixed, and mRNA was visualized by FISH. Each point is the average and standard error of at least five independent experiments, each of which consist of 30–45 cells. (H) To determine whether TPR-depletion promotes the degradation of newly synthesized mRNAs, the ratio of ftz-Δi signal in the control and TPR-depleted cells were plotted over time. Note that the relative level of ftz-Δi in TPR-depleted cells decreases over the first hour until about 60% of the mRNA remains, after which point the ratio is stable. (I) Cytoplasmic/total RNA ratio was quantified in control and TPR-depleted cells. (J) Cells were microinjected with in vitro synthesized MHC-ftz-Δi RNA, which was capped and polyadenylated, along with 70kDa Oregon Green Dextran to mark the injected compartment. The microinjected control- or TPR-depleted U2OS cells were fixed after various time points post injection. mRNA export was monitored in nuclear injected cells by FISH and the cytoplasmic/total distribution was quantified as in (I). Each point is the average and standard error of at least four independent experiments, each of which consists of 20–40 cells. (K, L) TPR depletion causes nuclear accumulation of poly(A)+ RNAs, as visualized by oligo-dT FISH staining, scale bar = 10 μm. Representative images are shown in (K), and quantification is shown in (L) with each bar representing the average and standard error of at least eight independent experiments for Control- or ‘TPR A’ depleted cells and four independent experiments for ‘TPR D’ depleted cells, each experiment consisting of at least 30 cells.