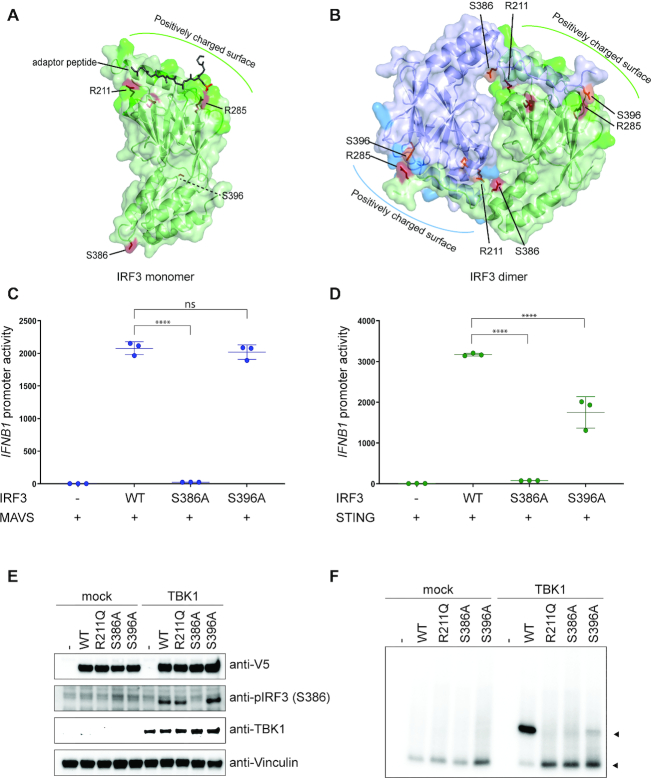
Figure 4.
The role of residue R211 in IRF3 dimerization. (A and B) Structural representation of IRF3 monomer (A) or dimer (B), where IRF3 is shown in green and blue, bound adaptor peptide is shown in dark green (A) and residues are highlighted in red and orange. (A and B) Figure was modeled on PDB ID: 5JEJ (A) and 5JEM (B). (C and D) IRF3-deficient HEK293T cells were transiently transfected with IFNB1 promoter firefly luciferase reporter, a constitutively active Renilla luciferase reporter and either WT IRF3 or mutant IRF3 as indicated. The cells were stimulated with MAVS (C) or cGAS and STING (D) and luciferase activities were measured 24 h post transfection. Firefly luciferase activity was normalized to Renilla luciferase activity and presented as triplicates ±SD. One-way ANOVA test was used for statistical analysis. ns, not significant; ****, P ≤ 0.0001. Similar data were obtained for two independent experiments (Supplementary Figure S6). (E and F) IRF3-deficient HEK293T cells were reconstituted with IRF3 WT or mutated IRF3 as indicated and stimulated with TBK1. Cell lysates were subjected to denaturing (E) or native PAGE (F) followed by immunoblotting with anti-V5 for total IRF3 (E and F), anti-IRF3 pS386, anti-STING or anti-Vinculin as a control (E).