Figure 1.
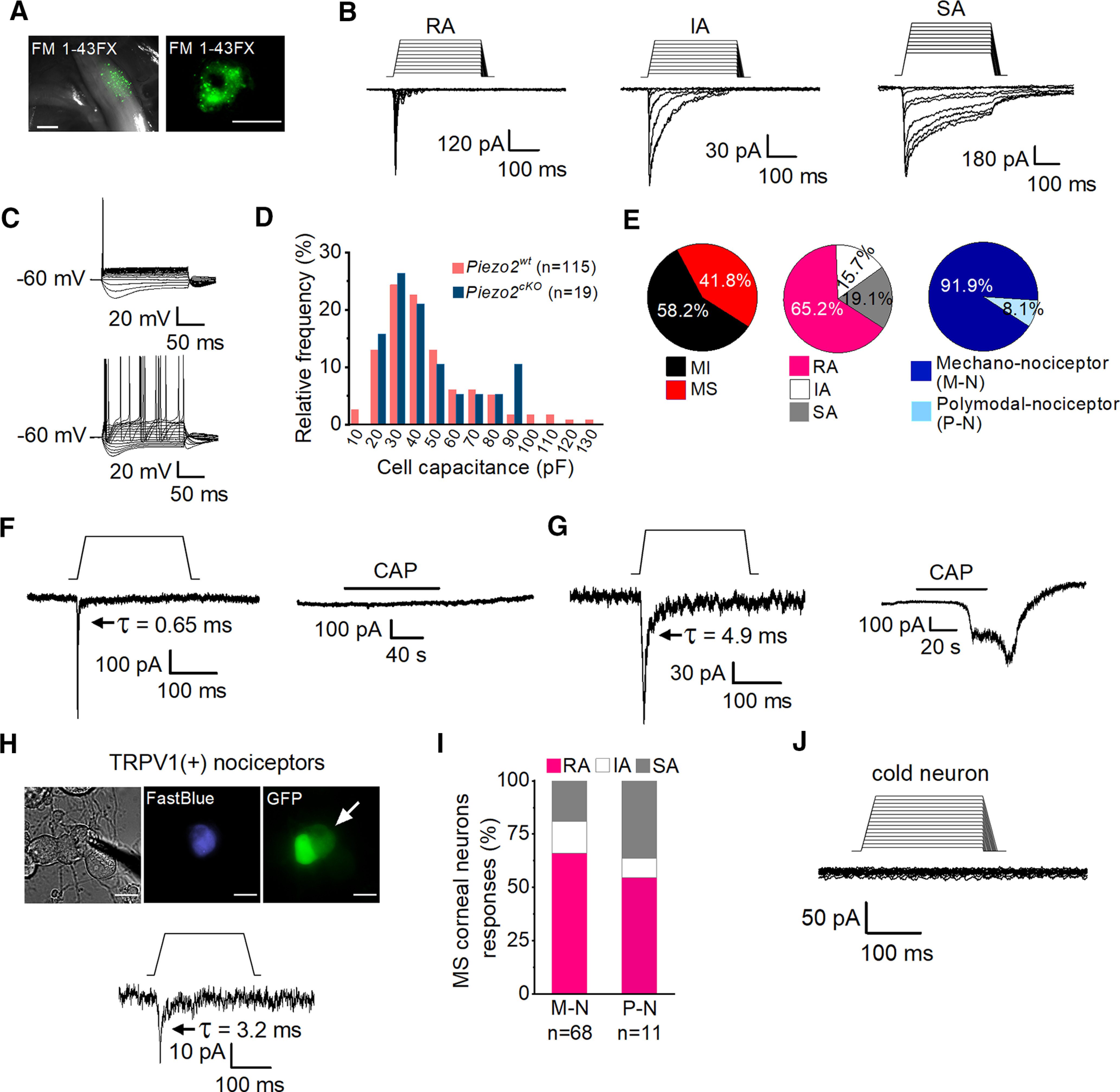
Mechanically activated currents in trigeminal corneal neurons. A, FM 1-43 FX fluorescent labeling of TG ganglia in fresh tissue (left) and of TG cultured cells (right) after its application to the corneal surface. Scale bars: left, 500 µm; right, 25 µm. B, Representative whole-cell recordings in the voltage-clamp configuration (Vhold = −60 mV) of the three general subtypes of mechanically activated currents, RA, IA, and SA, recorded from cultured corneal TG neurons identified by FM 1-43 FX labeling. Mechanical stimuli in 1 µm increments are indicated on top of each recording. Time constants of current relaxation are indicated in each panel. C, Representative whole-cell recordings in current-clamp configuration showing the phasic (n = 62, top) and tonic (n = 9, bottom) firing pattern of corneal TG neurons. D, Frequency histogram of cell capacitance shows that TG mechanically activated neurons are mainly of small and medium size and that the size distribution was similar for wild-type (pink) and Piezo2cKO mice (blue). E, Pie plots showing the distribution of mechano-sensitive (MS) (115 of 275) and mechano-insensitive (MI) corneal TG neurons (160 of 275; left); the proportion of the different types of mechanically activated currents is displayed with respect to the total number of MA recorded neurons (n = 115; central pie plot) and the distribution of the pure mechano-nociceptor (M-N; 68/74) and polymodal nociceptor (P-N; 6/74; right) neurons. F, G, Representative recordings of RA mechanically activated whole-cell currents (Vhold = −60 mV) of corneal neurons in response to mechanical indentation (left panels) and to 100 nm capsaicin (right panels). Mechanical stimuli of 250 ms duration are indicated on top of the recordings (11 µm and 7 µm respectively). H, Phase-contrast image (left) of a Fast Blue-labeled corneal neuron (middle) expressing TRPV1 (right, arrow), identified by the expression of GFP. Note that GFP labels two adjacent neurons. Scale bars, 25 µm. The bottom panel shows the whole-cell current response of the labeled neuron in the top panels to mechanically indentation (15 µm). The patch electrode in the phase-contrast image indicates the recorded neuron. I, Comparison of the proportions of MS corneal neurons responding with RA, IA, and SA currents in M-N (n = 68) versus P-N (n = 11); p = 0.682 (RA), p = 0.0.977 (IA), and p = 0.370 (Z-test). J, Representative traces of a whole-cell recording in the voltage-clamp configuration (Vhold = −60 mV) of a TRPM8-expressing cold-sensitive cultured corneal TG neuron identified by the expression of EYFP, showing the absence of a response to mechanical indentation. The mechanical stimulus in 1 μm increments is indicated on top of the recording. Time constants (τ) of MA current inactivation are indicated for each panel.
