Figure 8.
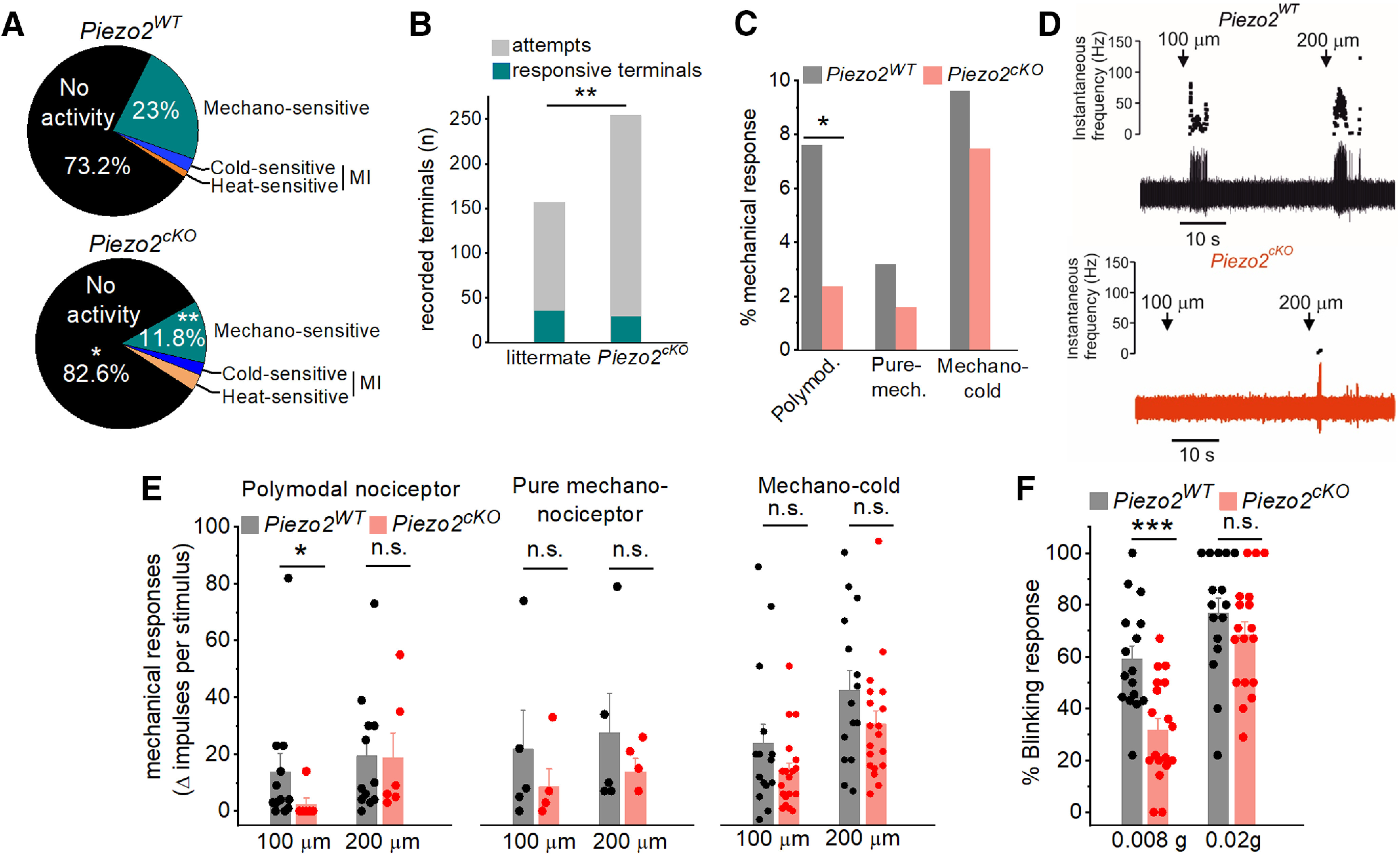
Piezo2 is involved in corneal nerve terminal mechanosensitivity. A, Distribution of the different functional classes of corneal terminals recorded in wild-type littermates (15 eyes from 9 animals) and Piezo2cKO mice (20 eyes from 15 animals; **p = 0.004; Z-test). B, Stacked bar plot shows significant differences in the successful attempts of recording a terminal from wild-type (157 corneal points) compared with Piezo2cKO (254 corneal points) mice. C, Proportion of corneal mechanosensitive terminals, identified as polymodal nociceptors, pure mechanoreceptors, and mechano-cold receptors from wild-type littermates (gray) and Piezo2cKO mice (light red; 5 pure-mechanoreceptors, 12 polymodal nociceptors, and 15 cold thermoreceptors in littermate mice; 4 pure mechanoreceptors, 6 polymodal nociceptors, and 19 cold thermoreceptors in Piezo2cKO mice; *p < 0.023; Z-test). D, Example of NTI activity in polymodal terminals from a wild-type littermate (top) and a Piezo2cKO mice (bottom) evoked by a 5 s forward displacement (100 and 200 µm) of the recording electrode. Arrows indicate the onset of the stimulus. The instantaneous frequency is represented at the top, and the original nerve impulse recording at the bottom. E, Histograms showing the increment of NTI activity of the different functional classes of corneal receptors in response to 100 and 200 µm displacement of the recording electrode in wild-type littermate and Piezo2cKO mice. Statistically significant differences were observed only in polymodal nociceptors in response to 100 µm (*p = 0.033; Student's t test, Mann–Whitney rank-sum test; n.s., not significant). F, Proportion of blink responses to the application of von Frey filaments (0.008 and 0.02 g) to the cornea in wild-type littermate (n = 16) and Piezo2cKO (n = 18) mice. ***p < 0.001 (Student's t test). n.s., not significant. E, F, Bars represent the averaged response, and the scattered symbols show the individual values of terminal recordings (E) or for each mice (F).
