Figure 13.
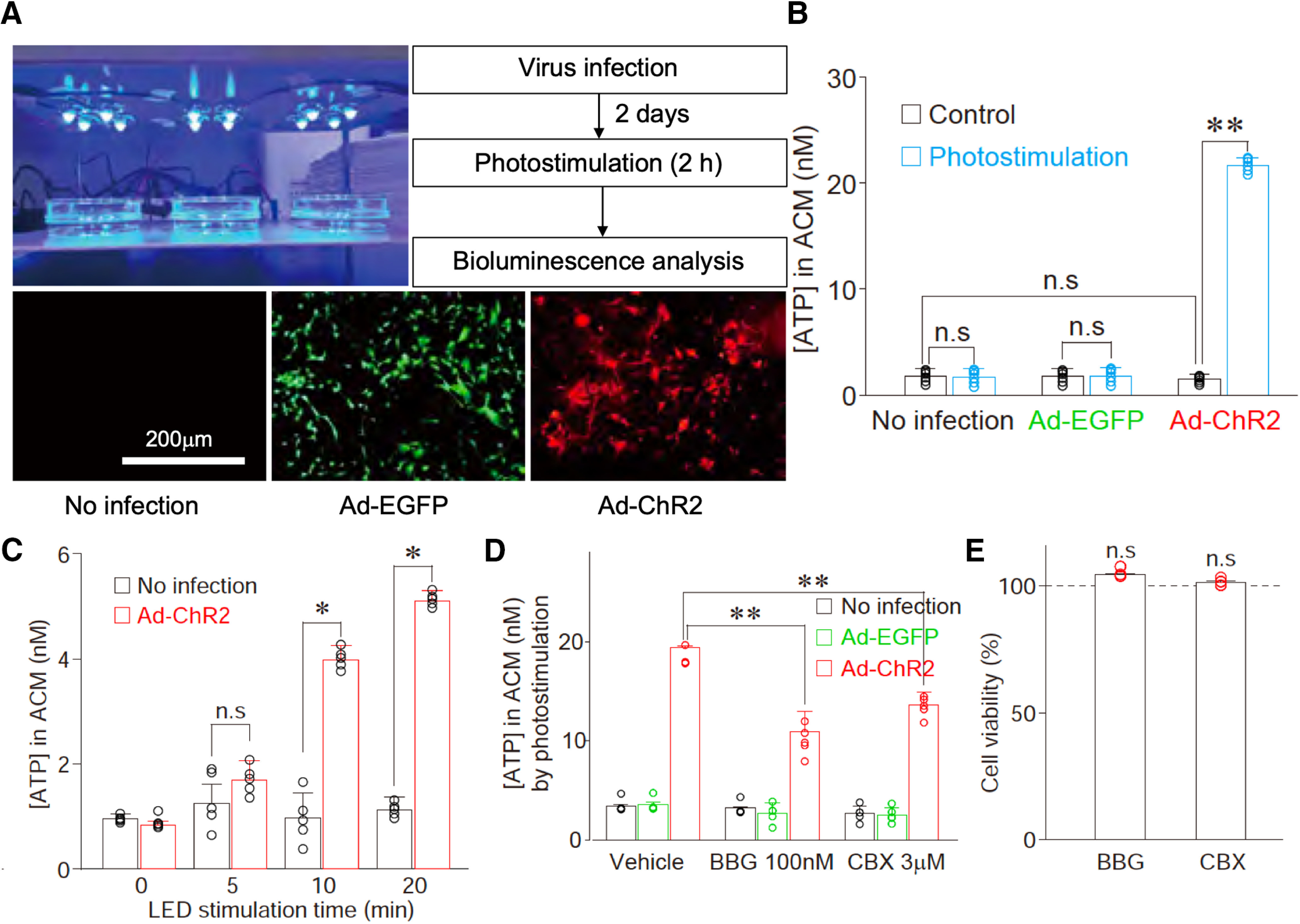
Optogenetic stimulation of cultured astrocytes induces ATP release. A, Primary astrocytes were infected with either Ad-EGFP (control virus, green, 7.6 × 1010 PFU/ml) or Ad-ChR2 (red). Two days after virus infection, cultured astrocytes were optically stimulated using an LED device before bioluminescence analysis of ATP release. The results are representative of three to six experiments. B, The extracellular ATP concentration was measured from the astrocyte-conditioned medium (ACM) in cultured astrocytes infected with either Ad-EGFP or Ad-ChR2 after 2-h photostimulation. Open circles represent the individual results (n = 6), and columns and error bars represent the mean and SD from six experiments; n.s., not significant; **p < 0.01; unpaired t test, t(10) = 29.50, p = 0.0001. C, The extracellular ATP concentration was measured from the ACM in cultured astrocytes infected with Ad-ChR2 by various duration of photostimulation. Open circles represent the individual results (n = 6), and columns and error bars represent the mean and SD from six experiments; n.s., not significant; **p < 0.01; unpaired t test, 10 min, t(10) = 8.67, p = 0.0001; 20 min, t(10) = 15.88, p = 0.0001. D, Effects of Brilliant Blue G (BBG, a P2X7 receptor antagonist, 100 nm) or CBX (a hemichannel blocker, 3 μm) on ATP release in cultured astrocytes. ChR2-expressing astrocytes were illuminated with LED in the absence (vehicle) or presence of 100 nm BBG or 3 μm CBX for 2 h. Extracellular ATP concentration was measured by a bioluminescence assay. Open circles represent the individual results (n = 5), and columns and error bars represent the mean and SD from five experiments; **p < 0.01; one-way ANOVA, F(2,12) = 29.42, p = 0.0001. E, Cell viability was measured by MTT assays 24 h after the treatment of 100 nm BBG or 3 μm CBX. Open circles represent the individual results (n = 4), and columns and error bars represent the mean and SD from four experiments; n.s.; not significant; one-way ANOVA.
