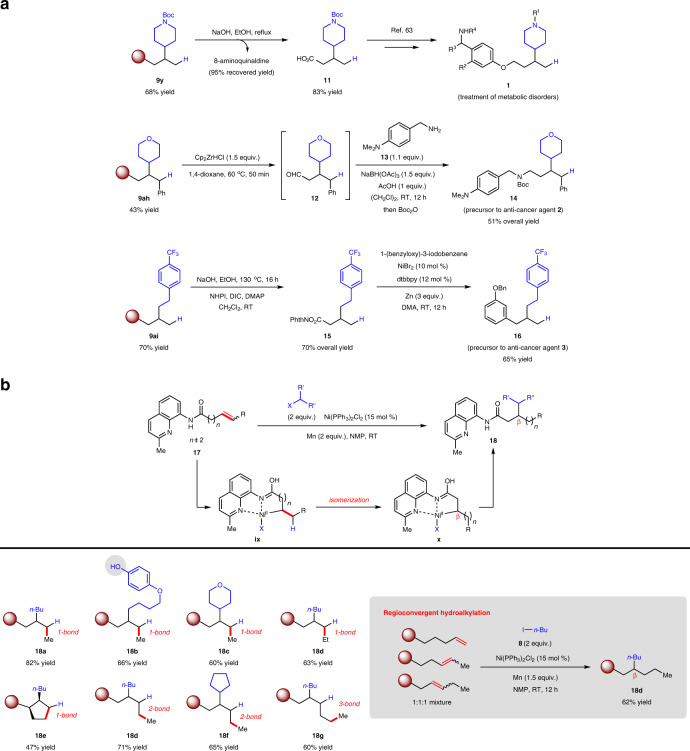
Fig. 4. Application to synthesis of biologically active molecules and remote hydroalkylation.
a The products resulting from reductive hydroalkylation can be conveniently transformed to a variety of medicinal compounds of interest. b Alkenyl amides bearing ≥ five-carbon chain length undergo remote hydroalkylation through in situ isomerization of alkylnickel intermediates, providing reliable access to β-alkylated molecules. Regioisomeric olefin mixtures can be regioconvergently converted to a single value-added product. Regioisomeric ratios were determined by 1H NMR analysis. Yields are for isolated and purified products. 9ah was obtained as a 96:4 regioisomeric mixture. For 18a, 18d, 18e, and 18g, reactions were conducted using iodides (X = I). For 18b, 18c, and 18f, reactions were conducted using bromides (X = Br). For 18g, 5 equiv. of 1-iodobutane was used. R, functional group; X, halide; NMP, N-methyl-2-pyrrolidone; DMA, N,N-dimethylacetamide; NHPI, N-hydroxyphthalimide; DIC, N,N′-diisopropylcarbodiimide; DMAP, 4-dimethylaminopyridine; dtbbpy, 4,4′-di-tert-butyl-2,2′-bipyridine; Boc, tert-butoxycarbonyl; Phth, phthaloyl; Cp, cyclopentadienyl; RT, room temperature.