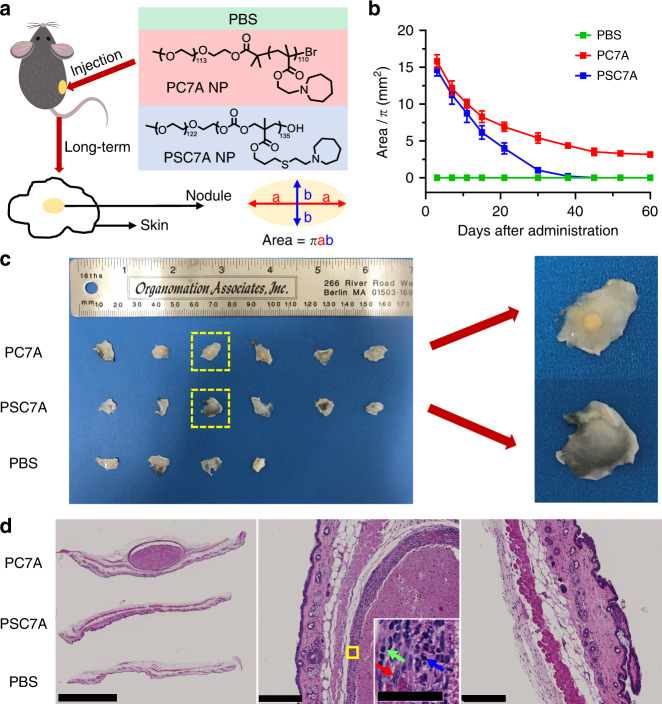
Fig. 7. Long-term safety evaluation of degradable PSC7A NP and non-degradable PC7A NP.
a C57BL/6 mice were subcutaneously injected with PBS, 300 μg PSC7A NP, or 300 μg PC7A NP on the right flank. Inflammatory nodules formed at the injection sites in PSC7A NP and PC7A NP groups. The surface areas of the nodules were calculated based on an ellipse model. b Change of nodule surface areas over time. n = 4 biologically independent mouse samples in PBS group, n = 6 biologically independent mouse samples in each PSC7A and PC7A group. Data are presented as means ± s.e.m. c Left: photographs of skin tissues taken from the injection sites on day 60, from top to bottom are mice treated with PC7A NP, PSC7A NP, and PBS. Right: magnification of skin tissues from PSC7A and PC7A groups. d Left: H&E staining of formalin-fixed, paraffin-embedded skin tissues (injection sites) on day 60, from top to bottom are mice treated with PC7A NP, PSC7A NP, and PBS. Scale bar: 2.5 mm. Middle: magnification of a nodule surrounded by granulomatous inflammation from PC7A group. Scale bar: 250 μm. Inset: magnification of the area marked by yellow square. The red, green, and blue arrows represent the macrophage, lymphocyte, and neutrophil, respectively. Scale bar: 50 μm. Right: magnification of skin tissue from PSC7A group. Scale bar: 250 μm. The whole set of experiment was repeated twice with similar results. In each experiment, two biologically independent mice were chosen randomly from each group on day 60. Skin tissues were harvested from each mouse and three adjacent sections of each sample were taken. One representative image from each group was shown.