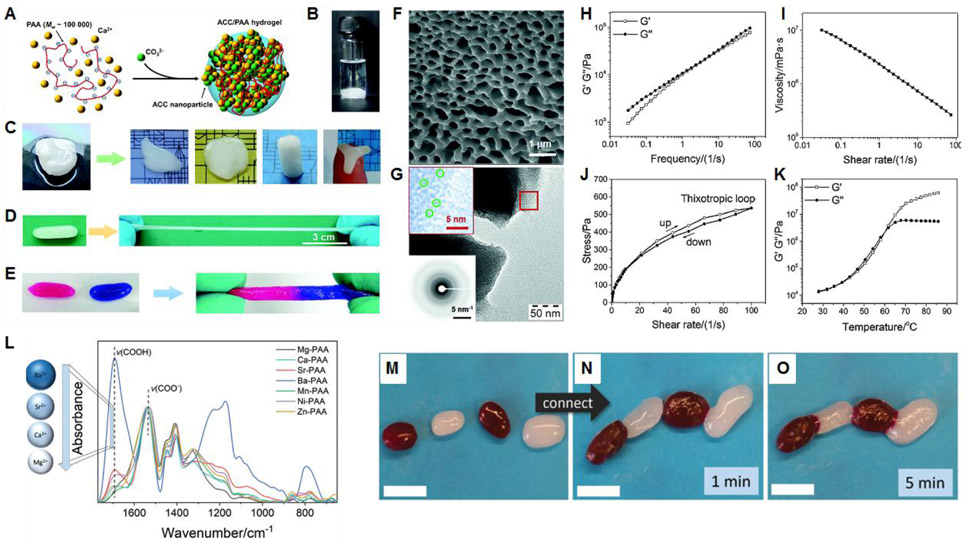
Figure 21.
(A) Schematic synthesis of the ACC/PAA supramolecular hydrogel. (B) ACC/PAA hydrogel is stable in water. (C) ACC/PAA hydrogel is plastic, which can be made in different shapes. (D) ACC/PAA hydrogel is stretchable. (E) Self-adhesion of ACC/PAA hydrogel. Dye molecules (rhodamine B and methylene blue) were introduced to produce the colors. (F) SEM image of the freeze-dried ACC/PAA hydrogel. (G) TEM images of ACC/PAA dry gel. The insets are the corresponding electron diffraction pattern and an enlarged view of the area highlighted by the red square illustrating the presence of very small ACC nanoparticles (highlighted by green circles), (H-K) Rheological behavior of the ACC/PAA hydrogel. (H) Frequency dependencies of the storage (G’) and loss (G’’) moduli. (I) Viscosity as a function of shear rate. (J) Thixotropic loop measurement. (K) Temperature dependencies of the storage (G’) and loss (G’’) moduli. Reprinted with permission from ref.394 Copyright 2016 WILEY-VCH. (L) The ATR–FTIR spectra of all mineral plastics indicate the complexation between carboxylates and respective metal ions (around 1530 cm−1) and the deprotonation degree of PAA (around 1700 cm−1). (M) Separated MnCO3/PAA hydrogels in the swollen state. The dark red color results from the addition of Toluylene Red, and (N and O) upon connection, the gels heal themselves within minutes. Reprinted with permission from ref.397 Copyright 2018 The Royal Society of Chemistry.