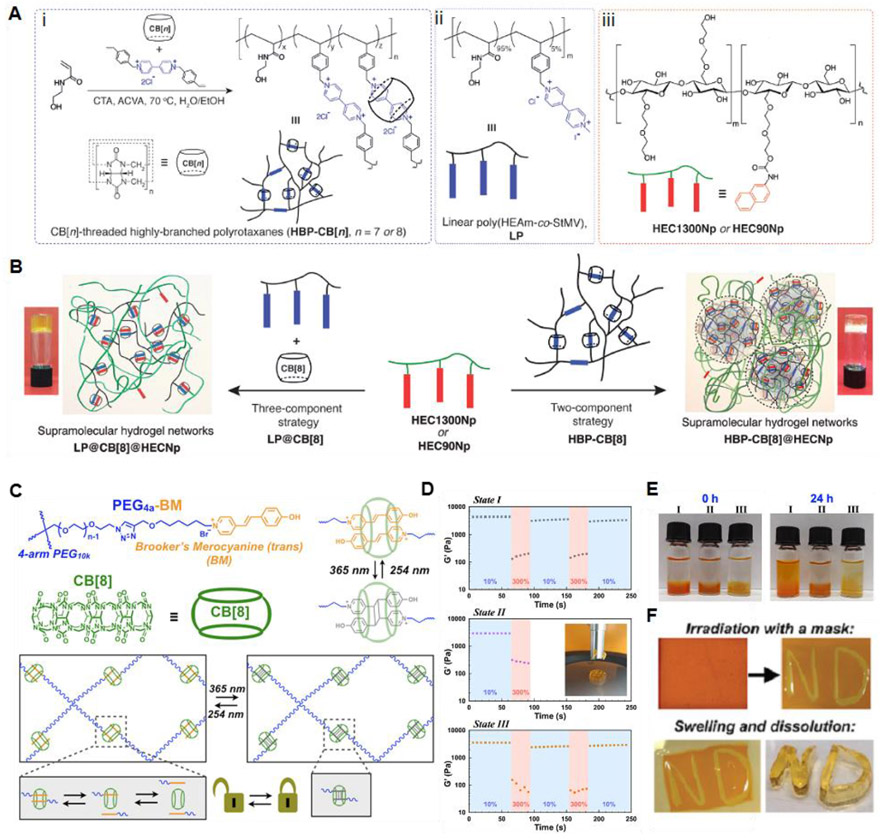
Figure 8.
(A) Schematic illustration of (i) synthesis of the highly branched CB[n]-threaded polyrotaxane (HBP-CB[n]) via a semi-batch RAFT polymerization in the presence of CB[n] (CTA : chain transfer agent (benzyltrithiocarbonyl propionic acid) and ACVA : 4,4′-azobis(4-cyanovaleric acid)), (ii) chemical structures of its linear analog (LP), and (iii) naphthyl-functionalized hydroxyethyl cellulose (HECNp). (B) Formation of hydrogel networks through a two-component strategy from HBP-CB[8] polyrotaxane (HBP-CB[8]@HECNp) or a three-component strategy from its linear analog (LP@CB[8]@HECNp). Inset: inverted vial tests for the hydrogel networks. Reprinted with permission from ref178. Copyright 2018 WILEY-VCH. (C) Light-controlled supramolecular hydrogels. (D) Step-strain rheology alternating between 10% staring and 300% strain for physically crosslinked hydrogels (State I), chemically cross-linked hydrogels (State II), or hydrogels with cross-links reversed by exposure to 254 nm irradiation (State III). (E) Hydrogel swelling and dissipation determined by bathing pre-formed hydrogels in water and hydrogel stability through vial inversion. (F) Hydrogels were patterned by irradiation with 365 nm light using a mask, and the remaining supramolecular network was dissolved in water to leave a patterned covalent hydrogel. Reprinted with permission from ref187. Copyright 2019 The Royal Society of Chemistry.