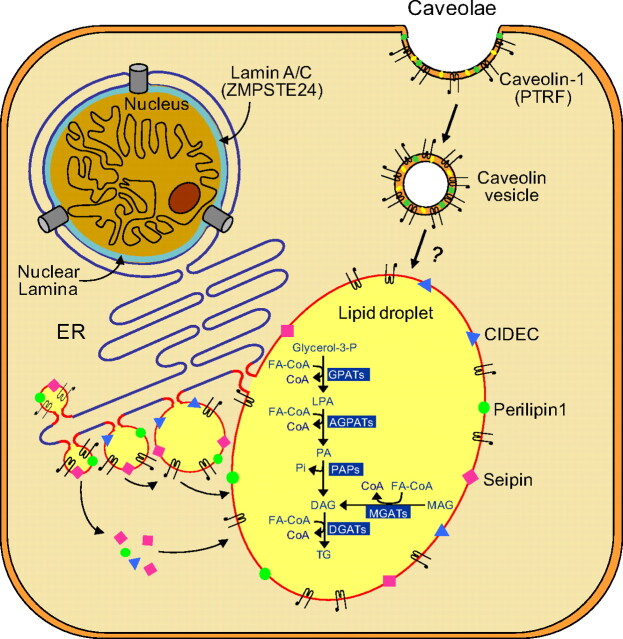
Fig. 2.
Lipid droplet formation in adipocytes. Lipid droplets (LD) are organelles that store triglycerides (TG) intracellularly. They form as budding vesicles at the endoplasmic reticulum (ER) that fuse in adipocytes to form one large LD. Many proteins, such as CIDEC (shown in blue triangles), seipin (pink squares), and perilipin 1 (green circles) are present on the LD membrane. CIDEC and seipin may be involved in fusion of LDs to form a larger LD, whereas perilipin 1 is essential for lipid storage and hormone-mediated lipolysis. Caveolae are formed from lipid rafts on the cell surface, which include cholesterol (yellow symbols), glycosphingolipids (green symbols), and caveolin-1 (black hairpin-like symbols). Endocytosis of caveolae forms caveolin vesicles that may directly merge with lipid droplets and thus translocating fatty acids to LDs. PTRF controls expression of caveolin 1 and 3 (data not shown). The classical and alternative pathways involved in the biosynthesis of TG are shown inside the lipid droplet. In the adipose tissue, TG synthesis requires glycerol-3-phosphate as the initial substrate (classical pathway), whereas in the small intestine, synthesis of TG can occur via an alternative pathway using monoacylglycerol (MAG) as the initial substrate. Acylation of glycerol-3-phosphate using fatty acyl coenzyme A (FA-CoA) at the sn-1 position is catalyzed by glycerol-3-phosphate acyltransferases (GPATs), resulting in the formation of 1-acylglycerol-3-phosphate or lysophosphatidic acid (LPA). LPA is then acylated at the sn-2 position by AGPATs to yield phosphatidic acid (PA). Removal of phosphate group from PA by PA phosphatases (PAP) produces diacylglycerol (DAG). Further acylation of DAG at the sn-3 position by diacylglycerol acyltransferases (DGATs) finally produces TG. In the alternative pathway, MAG is acylated to DAG by monoacylglycerol acyltransferases (MGATs) which is then further converted to TG. Lamin A/C are integral components of nuclear lamina (shown in blue color) and interact with nuclear membrane proteins as well as chromatin. Zinc metalloproteinase (ZMPSTE24) is critical for posttranslational processing of prelamin A to its mature form, lamin A. [Modified from A. Garg and A. K. Agarwal: Caveolin-1, a new locus for human lipodystrophy. J Clin Endocrinol Metab 93:1183–1185, 2008 (26), with permission. © The Endocrine Society. And from A. Garg and A. K. Agarwal: Lipodystrophies: disorders of adipose tissue biology. Biochem Biophys Acta 1791:507–513, 2009 (95), with permission. © Elsevier.]