Abstract
Aedes aegypti is the primary vector of dengue, Zika, yellow fever and chikungunya viruses to humans. In Africa, two subspecies, Ae. aegypti aegypti (Aaa) and Ae. aegypti formosus (Aaf) have been described. Until very recently, it was considered that the two forms were sympatric in East Africa and that only Aaf was present in Central and West Africa. However, recent data suggests that Aaa was also common in Senegal without any clear evidence of genetic differences with Aaf. This study was carried out in different Ae. aegypti populations from Senegal to better clarify their taxonomic status. The larvae, pupae and eggs were collected between July and September 2018 and reared individually to adult stage. For each population, F1 progeny from eggs laid by a single female F0 were reared as sibling samples. The number of pale scales on the first abdominal tergite (T1) and the basal part of the second tergite (T2) were counted. Individuals with no pale scale on T1 were classified as Aaf while those with at least one pale scale on this tergite were classified as Aaa. The morphological variations within families of Aaf were studied across 4 generations. In total, 2400 individuals constituting 240 families were identified, of which 42.5% were heterogeneous (families with both forms). Multivariate statistical analysis of variance including T1 and T2 data together showed that populations were significantly different from each other. Statistical analysis of T1 alone showed a similarity between populations from the southeast while variations were observed within northwest population. The analysis of family composition across generations showed the presence of Aaa and Aaf forms in each generation. The classification of Ae. aegypti into two subspecies is invalid in Senegal. Populations exhibit morphological polymorphism at the intra-family level that could have biological and epidemiological impacts.
Introduction
Zika (ZIKV), dengue (DENV), yellow fever (YFV) and chikungunya (CHIKV) viruses are transmitted mainly by Aedes aegypti worldwide. These arboviruses have experienced a significant geographic expansion, causing epidemics in different countries of Africa, Indian Ocean, Asia, Pacific, Europe and America despite all the considerable efforts made for their control [1, 2]. DENV is the most prevalent arthropod-borne virus in the world. More than half of the world's population is exposed to dengue fever and the number of infections is estimated at 390 million each year [3]. The four serotypes (DENV1-4) have all been reported In Africa. Recently, epidemics of dengue 1 (DEN1) occurred in Senegal (2017 and 2018) and Burkina Faso (2019) [4]. A re-emergence of CHIKV has been observed [5] especially in Asia and Indian Ocean islands. ZIKV is the most frequently amplified arbovirus in Senegal [2, 6] and recently has risen to considerable notoriety worldwide [7]. Despite the availability of a highly effective vaccine YF outbreaks are still frequents in Africa [8], and has recently occurred in Democratic Republic of Congo and Angola from which imported cases have been reported in China [9, 10]. Forty-seven countries, including 34 in Africa and 13 in Central and South America, are endemic to YF [8, 11]. Without any specific treatment and licensed vaccines (with the exception of YF) against these arboviruses, vector control is the only way of effective prevention and control. However, this vector control requires very precise targeting of the populations actually involved in transmission and therefore better knowledge of their structuration. Ae. aegypti, the most important epidemic vectors of DENV, ZIKV, CHIKV, and YFV, is present in practically all tropical and intertropical areas especially between 35° North and 35° South latitudes [12]. It is genetically the best characterized species in the genus Aedes [13]. This species presents great morphological and behavioral variability, close proximity to humans and the ability to transmit many pathogens [14, 15]. The first observation of morphological variations in Ae. aegypti was made by Hill in 1921 in Queensland, Australia [16]. This author noted that populations of Ae. aegypti which bred in the bush was darker than that associated with urban environment. This implicit correlation between differences in color and behavior was among the many concerns that prompted Mattingly to reassess the biology and taxonomy of Ae. aegypti [14, 17]. Considering the morphological, ecological and ethological data, he described a pale anthropophilic form which breeds in urban environment and a dark or wild form preferring natural breeding sites and animals for blood meals. Following this correlation between the habitat, the morphology and the behavior of the females, this author conventionally subdivided the species into two subspecies: Ae. aegypti aegypti (Aaa) and Ae. aegypti formosus (Aaf). Aaa was considered as domestic and anthropophilic with at least one pale scale on the first abdominal tergite. As for, Aaf was described as darker and characterized by the total absence of pale scales on this first abdominal tergite. This form Aaf was supposed to be present only in Africa with sylvatic and rather zoophilic behaviors. Referring to Mattingly's work, McClelland proposed a classification of the different forms based one the coloration encountered, ranging from the black "F" form to the palest form "R" [15]. Applying this classification to a large population of Ae. aegypti from around the world, this author questioned the notion of subspecies as defined by Mattingly [14] and proposed the possibility of an incipient speciation [18]. Until very recently, it was considered that the two forms were sympatric in East Africa without reproductive barrier [19] and only Aaf would be present in Central and West Africa [14, 20]. However, recent data suggests that Aaa was also common in Senegal and presented a northwest–southeast cline with a dominance of Aaa in the northwest and Aaf in the southeast [21, 22]. Unlike morphological and bioecological data, the analysis of the genetic differentiation of Ae. aegypti populations from different localities in Senegal provides no clear evidence of the existence of two genetically distinct groups [21, 23]. In addition, genetic relationships highlighted by several molecular markers such as microsatellites and single nucleotide polymorphisms often do not match with morphological similarities [24]. Overall, these results improve understanding of the diversity of Ae. aegypti in West Africa, but so far, all these studies were done at population level and none has been interested in intra-family morphological variations. However, a study on the polymorphism within 196 families (each coming from a single female) from 18 anthropophilic and non-anthropophilic populations of Ae. aegypti collected from different localities in South Africa, showed that 60.2% of the families were heterogeneous containing both Aaa and Aaf individuals [25]. These results suggest that the classification of Ae. aegypti into two subspecies is not valid in South Africa. A similar study in West Africa could help to explain the lack of genetic structuring of Ae aegypti subspecies in this area. It is in this context that we conducted this study on intra-family morphological polymorphism in different populations of Ae. aegypti from Senegal to better clarify on their taxonomic status.
Materials and methods
Sampling sites
Samples were collected between July and September 2018 in three climatic areas corresponding to three main rainfall area from south to north (Fig 1).
Fig 1. Collecting locations of Ae. aegypti populations, July-September 2018, Senegal.
This map was created using the R software (version 4.0.2) and the package rgdal using an empty shapefile from the HDX website (https://data.humdata.org/dataset/senegal-administrative-boundaries) available under Creative Commons Attribution 4.0 International license.
Kédougou, PK10, and Tambacounda are located in the south of the country and are undergoing demographic and economic changes. They have a Sudano-Guinean and Sudano-Sahelian climate. They are a crossroads of ecosystems characterized by a very diverse flora and fauna which are the result partly of favorable climatic characteristics. It is the rainiest area in the country (between 450 and 1300 mm/year from May to October) with temperatures ranging from 21°C to 42°C.
Dakar and Mbour are located in the west of the country dominated by a wooded savannah. They are among the most urbanized cities in Senegal. This area benefits from a coastal microclimate influenced by the trade winds and the monsoon. The relatively short hot and humid season lasts from July to October with mean temperatures around 27°C and annual rainfall of 300 to 400 mm/year.
Barkédji and Louga located in the northwest of the country are characterized by a dry Sahelian climate with a vegetation consisting of a savannah with trees and a long dry season of 9 months or more. The short and unstable rainy season records rainfall between 300 mm and 390 mm in 24 rainy days. Temperatures range between 21°C to 38°C and relative humidity between 30 and 75% [26].
All sampling sites in Kédougou, Tambacounda, Dakar, Mbour, Barkédji, and Louga were located in the center of urban areas in the domestic environment while the sampling site at PK10 were within a forest gallery in a sylvatic environment.
Field collection of samples
The samples were collected from various potential breeding sites of Ae. aegypti (Table 1). In the domestic environment, artificial breeding sites (used tires, bricks) were prospected to collect larvae and pupae. These immature stages were also collected in natural breeding sites (tree holes, fruit husks and rock holes) from one single forest gallery located at 10 km from Kédougou city (PK10). In this forest, the choice to study the variations of the two populations (PK10Aaf and PK10Aaa) was motivated by the first notification during our sampling of both forms (Aaf and Aaa) in sympatric in the natural breeding sites in contrast to previous data which only reported the presence of the Aaf form [21]. Eggs were collected with trap consisting of a black painted pot half filled with water in which an oblique piece of wood immersed at 2/3 was used as a laying substrate. These traps were hung in shaded places in the urban environment as well as in the forest. The samples were collected from sites at least 100 m apart.
Table 1. Breeding sites and collection stages of Aedes aegypti populations by locality, July-September 2018, Senegal.
| Locality | Latitude N | Longitude W | Breeding sites | Stages collected | Morphology of F0 |
|---|---|---|---|---|---|
| Kédougou | 12°33’45.3” | 12°10’31.9” | Used tires, bricks | Larvae + pupa | Aaf |
| PK10 forest | 12°36’43” | 12°14’46.80” | TH, RH, FH, Ovitraps | Larvae + pupa | Aaa, Aaf |
| Tambacounda | 14°9’52.73” | 14°5’8.98” | Used tires | Larvae + pupa | Aaf |
| Louga | 15°37’16.5” | 16°14’7.6” | Ovitraps | Eggs | Aaa |
| Barkédji | 15°16’37.4” | 15°51’46.8” | Ovitraps | Eggs | Aaa |
| Mbour | 14°25’7.6” | 16°57’23.1” | Used tires, poultry waterers | Larvae + pupa | Aaa |
| Dakar | 14°40’22.5” | 17°26’36.9” | Used tires, flower pots | Larvae + pupa | Aaa |
TH = Tree holes, RH = Rock holes, FH = Fruit husks.
Ethics statement
No specific permits were required for this study. No specific permissions were required for these activities and the locations investigated are not protected. This study did not involve endangered or protected species. The study protocol was carefully explained to the heads and inhabitants of each household investigated to obtain their informed oral consent.
Sample processing in the laboratory
Larvae and eggs were maintained under standard insectarium conditions [27] (temperature of 27± 1° C, relative humidity of 80% and a photoperiod of 12: 12h) until pupal stage. These pupae, as well as those collected directly in the field, were individually placed in test tubes.
After their emergence, adult mosquitoes (F0) were identified morphologically according to the descriptions of Mattingly and Huang [14, 28] and then grouped by sex. Subsequently, they were gently cold-anesthetized and their wings were spread using needles to check the presence or not of pale scales on the first abdominal tergite under binocular dissecting microscope. After identification, male and female individuals of the same form were pooled according to their origin for mating. The Aaa forms were chosen in Dakar, Mbour, Barkedji and Louga for the F0 parents while Aaf was chosen in Tambacounda and Kédougou and both forms in PK10 forest (Table 1).
F0 families egg batches production
For each population, 30 fully engorged Aaa or Aaf females were selected for individual egg batches production. Each female was placed in a cup covered with a mosquito net and a cotton wool soaked in water were deposited at the bottom to collect the eggs. These females were subsequently fed with 10% sucrose and maintained under standard insectarium conditions as describe previously.
Morphology of F1 progeny
Each egg batch (family) was reared separately into adults, and 10 F1 females identified. For that, the wings of the mosquitoes were cut off at their base and theirs bodies fixed using a needle horizontally crossing the thorax. The number of pale scales on the first abdominal tergite (T1) and the basal part of the second tergite (T2) were counted under a binocular dissecting microscope (Motic ST-36C-6LED) at 40 times magnification.
To follow the morphological variations within families across 4 generations, egg batches were produced from 5 pairs of Aaf for each generation. The different egg batches were separately reared into adults and 10 females identified, as described previously, per egg batch.
Data analysis
Mosquito specimens without any pale scale on the T1 tergite were classified Aaf while those with one or more pale scales on this T1 tergite were classified Aaa. Thus, families in which all individuals had the same forms (Aaa or Aaf) were considered as homogeneous while families which presented both forms (Aaa and Aaf) were considered as heterogeneous. The mean numbers of pale scales on both tergites (T1 + T2) of the different populations were compared by multivariate analysis of variances using the Wilk’Lambda test. The mean numbers of pale scales on each tergite (T1 and T2) were compared using the Waller-Duncan t-test [29]. The relative abundance of the two forms across the generations was compared with the χ2 test. Statistical analyses were performed using the R software version 2.15.1 [30] and results were considered significant when P < 0.05.
Results
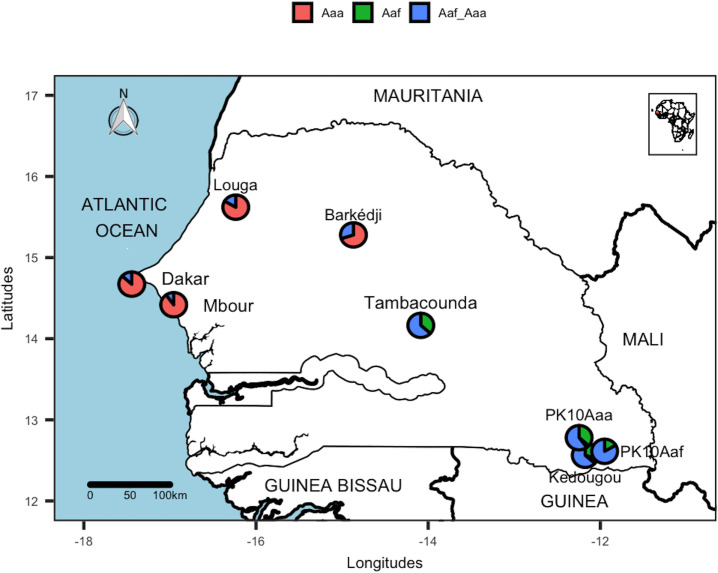
A total of 2400 female progenies belonging to 240 families were identified and the number of pale scales on their T1 and T2 counted. Out of the 240 families, 42.5% (102/240) and 15% (36/240) were respectively Aaa and Aaf homogeneous families. The remaining families (42.5% of the 240 families investigated) were heterogeneous, containing both the Aaa and Aaf forms (Table 2). For each population studied, part of the F1 offspring were morphologically different from their F0 parents. Populations from the southeast (Kédougou, PK10 and Tambacounda) presented higher heterogeneity rates (Fig 2) compared to those from the northwest (Dakar, Mbour, Louga and Barkédji) (P <0.05).
Table 2. Comparison of the average numbers of pale scales on T1 and T2 tergites and classification of F1 families in 8 Ae. aegypti populations, July-September 2018, Senegal.
| T1 | T2 | Number of families | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | Nb | min-max | Mean | sd | p-value | min-max | Mean | sd | p-value | Aaa | Aaf | Aaf+Aaa |
| Barkédji | 300 | 0–122 | 40.09 | 22.04 | a | 0–68 | 17.62 | 12.76 | a | 21 | 0 | 9 |
| Dakar | 300 | 0–83 | 25.77 | 16.31 | b | 0–76 | 8.13 | 10.13 | b | 26 | 0 | 4 |
| Louga | 300 | 0–124 | 31.76 | 18.18 | c | 0–42 | 16.23 | 10.43 | a | 25 | 0 | 5 |
| Mbour | 300 | 0–87 | 31.89 | 14.45 | c | 0–04 | 12.00 | 8.49 | c | 27 | 0 | 3 |
| PK10Aa | 300 | 0–35 | 2.36 | 5.03 | d | 0–17 | 1.56 | 2.91 | df | 1 | 11 | 18 |
| Kédougou | 300 | 0–23 | 2.66 | 4.72 | d | 0–23 | 0.92 | 2.56 | d | 2 | 9 | 19 |
| PK10Af | 300 | 0–32 | 1.79 | 4.37 | d | 0–16 | 6.89 | 2.77 | eb | 0 | 5 | 25 |
| Tambacounda | 300 | 0–34 | 2.39 | 6.04 | d | 0–23 | 3.35 | 5.18 | f | 0 | 11 | 19 |
| Total | 2400 | 102 | 36 | 102 | ||||||||
| 42.50% | 15% | 42.50% | ||||||||||
Identical letters indicate populations with a comparable average number of scales. Nb, number of individuals examined; sd, standard deviation; min, minimum number of scales; max, maximum number of scales; Aaa, homogeneous family Ae. aegypti aegypti; Aaf, homogeneous family Ae. aegypti formosus; Aaf + Aaa, heterogeneous family.
Fig 2. Proportion of homogeneous Aaa, homogeneous Aaf and heterogeneous (Aaf + Aaa) families of Ae. aegypti populations, July-September 2018, Senegal.
Two populations of Ae. aegypti from the Aaa (PK10Aaa) and Aaf (PK10Aaf) parents were investigated in the PK10 forest Site. This map was created using the R software (version 4.0.2) and the package rgdal using an empty shapefile from the HDX website (https://data.humdata.org/dataset/senegal-administrative-boundaries) available under Creative Commons Attribution 4.0 International license.
The Waller-Duncan t-test on T1 showed a similarity between Ae. aegypti populations from Kédougou, PK10 and Tambacounda (Table 2) with an average number of 1 to 2 pale scales on T1 (p> 0.05). However, variations were noted within Ae. aegypti populations from northwest sites with on average of pales scales ranging from 31 to 40. The population from Barkédji showed more pale scales on T1 (p <0.0001) whereas that from Dakar presented fewer pale scales on T1 than the others (p <0.0001). On the other hand, the populations from Mbour and Louga were comparable (p> 0.05). All of these populations had more pale scales on T1 than those from the southeast (p <0.0001).
The same analysis made on T2 showed significant variations between populations from the southeast and those from the northwest with the exception of Dakar and PK10Aaf which were comparable (Table 2). A similarity was noted between populations from Barkédji and Louga (p > 0.05) and between those from Kédougou and Tambacounda (p > 0.05).
When both tergites means (T1 + T2) were compared together by multivariate analysis, significant variations were observed across the 8 populations studied (p < 0.000 1).
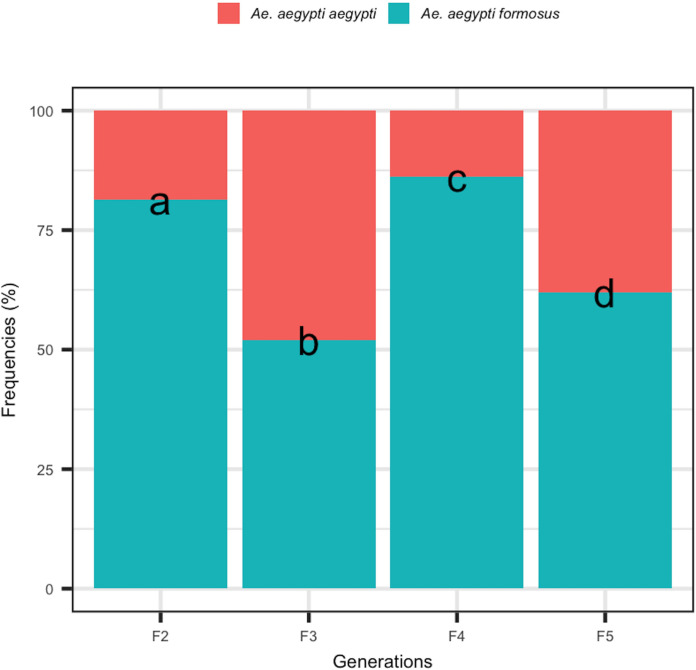
The analysis of the composition of the offspring of Aaf families across 4 generations showed that the two forms (Aaa and Aaf) were present in each generation (Fig 3). The relative abundance of the two forms was statistically different from one generation to another (p <0.05).
Fig 3. Relative abundance of Aaa and Aaf forms across four generations (F2 to F5) of Aaf parents.
Different letters indicate a significant difference from one generation to another.
Discussion
This study conducted on 2400 females belonging to 240 families, showed significant intra-family morphological variations in different populations of Ae. aegypti from Senegal. Our data showed that a part of the F1 progeny was morphologically different from their F0 parents whatever the population studied. This intra-family heterogeneity in the offspring noted during this study suggest that the classification of Ae. aegypti into two subspecies by Mattingly [14] based on the presence of pale scales on the first abdominal tergite (T1) should be considered as invalid in Senegal. The presence of both forms (Aaf and Aaa) across the country (at family level) is discordant with an earlier study which showed a southeast/northwest cline in the distribution of these two forms at population level with the exclusive presence of Aaf in the southeast, Aaa in the northwest and both forms in the center of the country [21]. The non-detection of Aaa in the southeast and Aaf in the northeast, in this previous study, could be explained by their low intra-family proportions in the populations studied and the possible influence of some factors (temperature, relative humidity, rainfall, etc.) favoring a high mortality of the less represented forms. The impact of these factors could explain the southeast/northwest cline of Aaa and Aaf as previously observed [21]. Comparative studies on the survival of both forms from the southeast, center and northeast of Senegal are necessary to assess the possible impact of these factors. Our results are similar to those obtained during a study of the polymorphism of Ae. aegypti populations in South Africa [25]. Indeed, the author by rearing separately the progenies of several females of Aaf or Aaa has observed the presence of both forms in several families. As in South Africa, the proportion of homogeneous Aaf families was lower than that of the homogeneous Aaa and the heterogeneous families. However, the proportion of homogeneous Aaf families was more important in Senegal. These intra-family morphological variations could explain the significant variations observed within and between different Ae. aegypti populations worldwide. These variations also explain the presence in sympatry of both forms, formerly considered as 2 different subspecies, in sub-Saharan Africa from natural and artificial breeding sites [14, 21, 31–33]. It is interesting to note that our results are in perfect agreement with all the genetic studies which did not show any clear differentiation between individuals belonging to the two forms collected in several localities of Senegal [21–23, 34, 35]. The intra-family morphological variations of Ae. aegypti populations in other parts of Africa should be systematically reviewed to determine their taxonomic status. The heterogeneity rates in the progeny were higher in the populations from the southeast (Kédougou, PK10 and Tambacounda) compared to those from the northwest (Dakar, Mbour, Louga and Barkédji). That could be explained by an increase of Aaa specimens in this area in correlation with the beginning of an adaptation to the domestic environment of these so-called wild populations. Consistent with these data, a recent study showed the existence of a highly anthropophilic neo-population of Ae. aegypti in this area (unpublished data). Based on the average number of pale scales on T1 tergite, our results showed a similarity in populations from the southeast (Kédougou, PK10 and Tambacounda). This could be explained by a conservation of sylvatic characters, in particular the dark coloring of tergites in these populations evolving under the same ecological conditions and dominated by the Aaf form [21, 23]. Indeed, an impact of climatic factors including temperature and relative humidity on the geographic distribution of the two forms has been noted by some authors [32, 36]. In agreement with other studies, our results showed variations in coloration on the basal part of T2 ranging from complete absence to well-marked bands [15, 25]. These variations less reflected the geographic distribution of the two forms in Senegal compared to T1.
The presence of both forms across the four generations of Aaf parents seems to confirm that the coloring of scales on T1 is a polymorphic morphological character within families. Ae. aegypti should be viewed as a highly polymorphic rather than a polytypic species.
Other studies reported chromosomal inversions in Aaf from Senegal [37] and elsewhere with genetic introgression between Aaa and Aaf [38] suggesting a chromosomal polymorphism in Ae. aegypti populations. These chromosomal inversions have been directly associated with behaviors such as feeding behavior [39, 40], oviposition site preferences [41, 42], insecticide resistance [43] and immune response to parasites [44, 45] in malaria mosquito vectors. Moreover, mutant markers for abdominal coloring have been reported on Ae. aegypti chromosomes [13, 46].
Many studies showed that Aaa populations have higher vector competence compared to Aaf [21, 47]. This difference in vector competence could be linked to differences in competence of individuals of both forms. Thus, to test this hypothesis, it would be interesting to compare the intra-family variations in vector competence of individuals belonging to the Aaf and Aaa forms.
Conclusion
This study revealed a morphological polymorphism at intra-family level in different populations of Ae. aegypti from Senegal. The presence of pale scales on T1 as a classification criterion for the two forms should be considered as invalid in Senegal. However, this study reveals two distinct groups; a group located in the southeast of the country with an average of 1 to 2 pale scales on T1 and another group in the northwest with an average of 31 to 40 pale scales on same tergite. Additional detailed chromosome and/or genomic studies could give more explanation to these intra-family and inter-population morphological variations which could have an impact on biological parameters and the transmission of pathogens by Ae. aegypti in Senegal.
Acknowledgments
The authors would like to thank Mamoudou Ba, Oumar Ba, Moussa Ba and Ibrahima Niang for their technical assistance and collaboration.
Data Availability
All data generated or analyzed during this study are included in this published article.
Funding Statement
This study was supported fully by H2020-INFRAIA-2016-2017 (INFRAVEC2) funds. There was no additional external funding received for this study.
References
- 1.Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2: 789–801. 10.1038/nrmicro1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diallo D, Dia I, Diagne CT, Gaye A, Diallo M. Emergences of Chikungunya and Zika in Africa Chikungunya and Zika Viruses. Elsevier; 2018. pp. 87–133. [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO AFRO Outbreaks and Other Emergencies, Week 44: 28 October—03 November 2017 (Data as reported by 17:00; 03 November 2017)—Senegal. In: ReliefWeb [Internet]. 2017 [cited 26 Mar 2020]. Available: https://reliefweb.int/report/senegal/who-afro-outbreaks-and-other-emergencies-week-44-28-october-03-november-2017-data
- 5.Vasconcelos PFC, Powers AM, Hills S. The Emergence of Chikungunya and Zika Viruses in the Americas Chikungunya and Zika Viruses. Elsevier; 2018. pp. 215–235. [DOI] [Google Scholar]
- 6.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One. 2014;9: e109442 10.1371/journal.pone.0109442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diallo D, Diallo M. Why is Zika virus so rarely detected during outbreaks and how can detection be improved? BMC Res Notes. 2017;10: 524 10.1186/s13104-017-2854-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diallo D, Sall AA, Diagne CT, Faye O, Hanley KA, Buenemann M, et al. Patterns of a sylvatic yellow fever virus amplification in southeastern Senegal, 2010. Am J Trop Med Hyg. 2014;90: 1003–13. 10.4269/ajtmh.13-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed QA, Memish ZA. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47: 92–96. 10.1177/0049475517699726 [DOI] [PubMed] [Google Scholar]
- 10.Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med. 2017;24 10.1093/jtm/tax008 [DOI] [PubMed] [Google Scholar]
- 11.WHO. Yellow fever. 2019 [cited 25 Mar 2020]. Available: https://www.who.int/news-room/fact-sheets/detail/yellow-fever
- 12.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Aedes albopictus. Jit M, editor. eLife. 2015;4: e08347 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severson DW, Mori A, Zhang Y, Christensen BM. Linkage Map for Aedes aegypti Using Restriction Fragment Length Polymorphisms. J Hered. 1993;84: 241–247. 10.1093/oxfordjournals.jhered.a111333 [DOI] [PubMed] [Google Scholar]
- 14.Mattingly PF. Genetical Aspects of the Aedes aegypti Problem: I.—Taxonomy and Bionomics. Ann Trop Med Parasitol. 1957;51: 392–408. 10.1080/00034983.1957.11685829 [DOI] [PubMed] [Google Scholar]
- 15.McClelland GAH. A Preliminary Study of the Genetics of Abdominal Colour Variations in Aedes aegypti (L.) (Diptera, Culicidae). Ann Trop Med Parasitol. 1960;54: 305–320. 10.1080/00034983.1960.11685991 [DOI] [Google Scholar]
- 16.Hill GF. Notes on Some Unusual Breeding-Places of Stegomyia Fasciata, F abr., in Australia. Ann Trop Med Parasitol. 1921;15: 91–92. 10.1080/00034983.1921.11684253 [DOI] [Google Scholar]
- 17.Mattingly PF. Genetical Aspects of the Aedes aegypti Problem: II. Disease Relationships, Genetics and Control. Ann Trop Med Parasitol. 1958;52: 5–17. [PubMed] [Google Scholar]
- 18.McClelland GAH. A worldwide survey of variation in scale pattern of the abdominal tergum of Aedes aegypti (L.)(Diptera: Culicidae). Trans R Entomol Soc Lond. 1974;126: 239–259. [Google Scholar]
- 19.Moore DF. Hybridization and mating behavior in Aedes aegypti (Diptera: Culicidae). J Med Entomol. 1979;16: 223–226. 10.1093/jmedent/16.3.223 [DOI] [PubMed] [Google Scholar]
- 20.Kröpelin S, Verschuren D, Lezine A-M, Eggermont H, Cocquyt C, Francus P, et al. Climate-Driven Ecosystem Succession in the Sahara: The Past 6000 Years. Science. 2008;320: 765–768. 10.1126/science.1154913 [DOI] [PubMed] [Google Scholar]
- 21.Sylla M, Bosio C, Urdaneta-Marquez L, Ndiaye M, Black WC. Gene Flow, Subspecies Composition, and Dengue Virus-2 Susceptibility among Aedes aegypti Collections in Senegal. Harris E, editor. PLoS Negl Trop Dis. 2009;3: e408 10.1371/journal.pntd.0000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paupy C, Brengues C, Ndiath O, Toty C, Hervé J-P, Simard F. Morphological and genetic variability within Aedes aegypti in Niakhar, Senegal. Infect Genet Evol. 2010;10: 473–480. 10.1016/j.meegid.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 23.Huber K, Dia I, Mathiot C, Sall AA, Diallo M. Aedes aegypti in Senegal: Genetic Diversity and Genetic Structure of Domestic and Sylvatic Populations. Am J Trop Med Hyg. 2008;79: 1003–13. [PubMed] [Google Scholar]
- 24.Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—A Review. Mem Inst Oswaldo Cruz. 2013;108: 11–17. 10.1590/0074-0276130395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jupp PG, Kemp A, Frangos C. The potential for dengue in South Africa: morphology and the taxonomic status of Aedes aegypti populations. Mosq Syst 1991;23: 182–190. [Google Scholar]
- 26.ANSD. Conseil Régional de Kédougou / Tambacounda / Dakar/Louga/Thiès.). 2016 [cited 4 Mar 2020]. Available: http://www.ansd.sn/index.php
- 27.Gerberg EJ, Barnard DR, Ward RA. Manual for mosquito rearing and experimental techniques. American Mosquito Control Association, Inc; Lake Charles; 1994. Available: https://www.cabdirect.org/cabdirect/abstract/19952010555 [Google Scholar]
- 28.Huang Y-M. The subgenus Stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae). Zootaxa. 2004;700: 1–120. 10.11646/zootaxa.700.1.1 [DOI] [Google Scholar]
- 29.Waller RA, Duncan DB. A Bayes Rule for the Symmetric Multiple Comparisons Problem. J Am Stat Assoc. 1969;64: 1484–1503. 10.1080/01621459.1969.10501073 [DOI] [Google Scholar]
- 30.R core Team. R: A language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria; 2016. [Google Scholar]
- 31.Futami K, Iwashita H, Higa Y, Lutiali PA, Sonye GO, Mwatele C, et al. Geographical Distribution of Aedes aegypti aegypti and Aedes aegypti formosus (Diptera: Culicidae) in Kenya and Environmental Factors Related to Their Relative Abundance. Wilkerson R, editor. J Med Entomol. 2019; 57: 772–779. tjz233. 10.1093/jme/tjz233 [DOI] [PubMed] [Google Scholar]
- 32.Higa Y, Abílio AP, Futami K, Lázaro MAF, Minakawa N, Gudo ES. Abundant Aedes (Stegomyia) aegypti aegypti mosquitoes in the 2014 dengue outbreak area of Mozambique. Trop Med Health. 2015;43: 107–109. 10.2149/tmh.2014-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabachnick WJ, Munstermann LE, Powell JR. Genetic distinctness of sympatric forms of Aedes aegypti in East Africa. Evolution. 1979;33: 287–295. 10.1111/j.1558-5646.1979.tb04682.x [DOI] [PubMed] [Google Scholar]
- 34.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, et al. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito: evolutionary genetic history of Aedes aegypti. Evolution. 2014;68: 514–525. 10.1111/evo.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallis GP, Tabachnick WJ, Powell JR. Macrogeographic genetic variation in a human commensal: Aedes aegypti, the yellow fever mosquito. Genet Res. 1983;41: 241–258. 10.1017/s0016672300021315 [DOI] [PubMed] [Google Scholar]
- 36.Machado-Allison CE, Craig GB. Geographic Variation in Resistance to Desiccation in Aedes aegypti and A. atropalpus (Diptera: Culicidae). Ann Entomol Soc Am. 1972;65: 542–547. 10.1093/aesa/65.3.542 [DOI] [Google Scholar]
- 37.Bernhardt SA, Blair C, Sylla M, Bosio C, Black IV WC. Evidence of multiple chromosomal inversions in Aedes aegypti formosus from Senegal. Insect Mol Biol. 2009;18: 557–569. 10.1111/j.1365-2583.2009.00895.x [DOI] [PubMed] [Google Scholar]
- 38.Redmond SN, Sharma A, Sharakhov I, Tu Z, Sharakhova M, Neafsey DE. Linked-read sequencing identifies abundant microinversions and introgression in the arboviral vector Aedes aegypti. BMC Biol. 2020;18: 1–14. 10.1186/s12915-019-0728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73: 483–497. 10.1016/0035-9203(79)90036-1 [DOI] [PubMed] [Google Scholar]
- 40.Main BJ, Lee Y, Ferguson HM, Kihonda A, Govella NJ, Kreppel KS, et al. The genetic basis of host preference and indoor resting behavior in the major African malaria vector, Anopheles arabiensis. bioRxiv. 2016; 044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manoukis NC, Powell JR, Touré MB, Sacko A, Edillo FE, Coulibaly MB, et al. A test of the chromosomal theory of ecotypic speciation in Anopheles gambiae. Proc Natl Acad Sci. 2008;105: 2940–2945. 10.1073/pnas.0709806105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanford MR, Ramsay S, Cornel AJ, Marsden CD, Norris LC, Patchoke S, et al. A preliminary investigation of the relationship between water quality and Anopheles gambiae larval habitats in western Cameroon. Malar J. 2013;12: 225 10.1186/1475-2875-12-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooke BD, Hunt RH, Coetzee M. Resistance to dieldrin+ fipronil assorts with chromosome inversion 2La in the malaria vector Anopheles gambiae. Med Vet Entomol. 2000;14: 190–194. 10.1046/j.1365-2915.2000.00222.x [DOI] [PubMed] [Google Scholar]
- 44.Petrarca V, Beier JC. Intraspecific chromosomal polymorphism in the Anopheles gambiae complex as a factor affecting malaria transmission in the Kisumu area of Kenya. Am J Trop Med Hyg. 1992;46: 229–237. 10.4269/ajtmh.1992.46.229 [DOI] [PubMed] [Google Scholar]
- 45.Riehle MM, Bukhari T, Gneme A, Guelbeogo WM, Coulibaly B, Fofana A, et al. The Anopheles gambiae 2La chromosome inversion is associated with susceptibility to Plasmodium falciparum in Africa. Elife. 2017;6: e25813 10.7554/eLife.25813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munstermann LE, Craig GB. Genetics of Aedes aegypti. J Hered. 1979;70: 291–296. 10.1093/oxfordjournals.jhered.a109261 [DOI] [Google Scholar]
- 47.Diallo M, Ba Y, Faye O, Soumare ML, Dia I, Sall AA. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans R Soc Trop Med Hyg. 2008;102: 493–498. 10.1016/j.trstmh.2008.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.