Fig. 1. Characterization of the interaction of 10074-G5 with monomeric Aβ42.
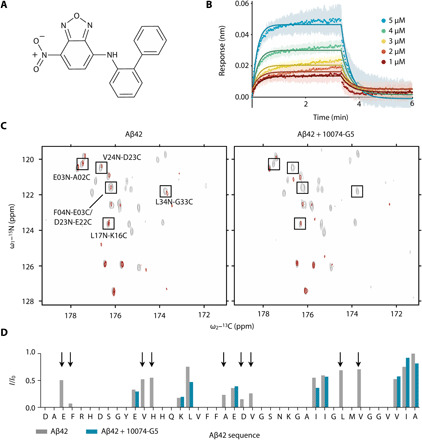
(A) Structure of biphenyl-2-yl-(7-nitro-benzo[1,2,5]oxadiazol-4-yl)-amine, also known as 10074-G5. (B) Biolayer interferometry measurements showing the dose-dependent binding of 10074-G5 to an Aβ42-functionalized surface at various concentrations of the added compound. The curves were corrected for baseline drift. Raw data are shown in fig. S1A. Control curves showing negligible nonspecific binding are shown in fig. S1 (B and D). Global fitting to simple one-phase association and dissociation equations yields association (kon) and dissociation (koff) rates to be 8.5 × 103 ± 0.2 × 103 M−1 s−1 and 4.7 × 10−2 ± 2 × 10−4 s−1, respectively, corresponding to a binding dissociation constant (Kd) of 6 μM. For this fit, all five curves were constrained to single, shared kon and koff values. The global R2 for the fits is 0.98. (C) 2D HN–BESTCON spectra in the absence (left) and presence (right) of 1:2 Aβ42:10074-G5 with (red) and without (gray) selective water presaturation, performed at 15°C. (D) Quantification of the relative I/I0 intensities from (C) shows that the peptide amide groups are more exposed to solvent in the presence of 10074-G5 (blue) as compared to its absence (gray). Arrows highlight regions along the sequence in which signals are detectable in the absence of the compound, but not in its presence, thus suggesting that 10074-G5 increases the solvent exposure of specific regions of Aβ42. ppm, parts per million.
