Fig. 4. 10074-G5 sequesters monomeric Aβ42 and inhibits its aggregation.
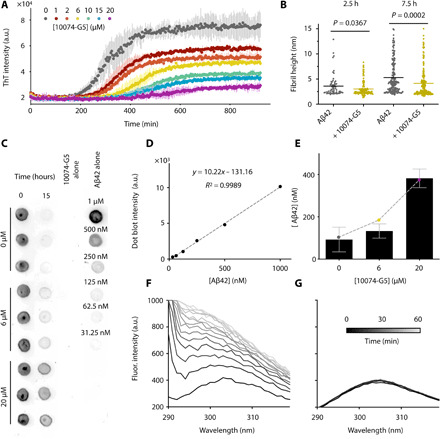
(A) ThT measurements using 1 μM Aβ42 show concentration-dependent effects of 10074-G5 on Aβ42 aggregation. Measurements were taken in triplicate. The concentration of DMSO was held constant across all samples. (B) Distributions of cross-sectional heights of 1 μM Aβ42 fibrils at 2.5 hours (n ≥ 60) and 7.5 hours (n ≥ 200) formed with and without 6 μM 10074-G5, from single-molecule analyses of AFM maps (fig. S7). Lines indicate means. P values were determined by unpaired, two-tailed Student’s t test. Fibrillar aggregates formed in the presence of 10074-G5 have smaller cross-sectional diameters than those formed in its absence. (C) Dot blot of soluble Aβ42 before and after the aggregation of 1 μM Aβ42 with and without 10074-G5 using the W0-2 antibody indicates sequestration of soluble Aβ42. Blotting was performed in triplicate. Fit (D) and quantification (E) used to estimate the concentration of soluble Aβ42 remaining at the end of the aggregation reaction from (C). The dashed curve in (E) represents fit to eq. S14, describing the equilibrium concentration of unreacted monomer from a competitive binding of free monomers to fibril ends and inhibitor. Using this simple fit, we determined the fitted affinity of 10074-G5 for the soluble material to be Kd = 7 ± 1 μM. Intrinsic fluorescence profiles of Tyr10 of 5 μM Aβ42 in the absence (F) and presence (G) of 1:1 10074-G5 over 1 hour show that 10074-G5 delays an increase in fluorescence, suggesting that 10074-G5 inhibits early aggregation events including oligomerization and multimerization. Error bars represent ± SDs in (A) and (E).
