Fig. 5. 10074-G5 inhibits Aβ42 aggregation primarily by monomer sequestration.
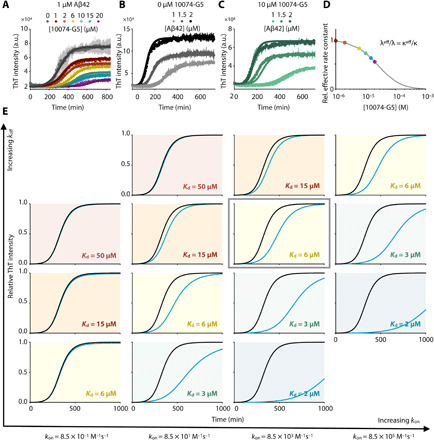
(A) Global fit of ThT kinetic curves to a monomer sequestration model (eq. S12), in which 10074-G5 affects the aggregation by binding free monomers. Measurements are those shown in Fig. 4A. The theoretical curves are obtained using eq. S11 with unperturbed kinetic obtained from (B) leaving Kd as the only global fitting parameter (eq. S15). The global fit yields Kd = 40 μM. Global fits to eq. S15 were performed on normalized data to extract changes in the rate parameters in the presence of 10074-G5. (B) Global fit to eq. S11 and eq. S12 of ThT kinetic traces of the aggregation reaction for increasing concentrations of Aβ42 (1, 1.5, and 2 μM) in the absence of 10074-G5. Measurements were taken in duplicate or triplicate. (C) Overlay of theoretical kinetic curves from (A) with independent ThT kinetic traces of the aggregation reaction for increasing concentrations of Aβ42 (1, 1.5, and 2 μM) in the presence of 10 μM 10074-G5. Solid curves are predictions of the kinetic monomer sequestration model using the same rate parameters and inhibitor binding constant as in (A) and no fitting parameters. Measurements were taken in duplicate or triplicate. (D) Effective rates of aggregate proliferation through primary (λ) and secondary (κ) nucleation in the presence of varying concentrations of 10074-G5 determined using the global fit in (A). Error bars represent ± SDs in (A to C). (E) Phase diagram illustrating numerical solutions to the kinetic equations for different kon and koff rates. Curves represent kinetic aggregation traces of 1 μM Aβ42 in the absence (black) and presence of 2 μM compound (blue). Diagonals correspond to constant values of Kd. The values of k+kn and k2k+ are the same as those shown in (A).
