Fig. 2. Defining the LMTK3 consensus phosphorylation motif and identifying HSP27 as an LMTK3 substrate.
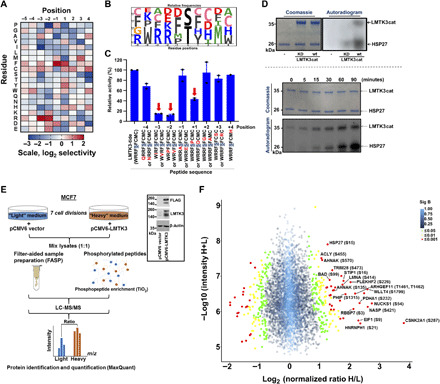
(A) A spatially 198 components arrayed PSPL was subjected to in vitro phosphorylation with active LMTK3cat. A representative image of the average log2 values of two independent experiments is shown. (B) Scaled-sequence PhosphoLogo representation of the LMTK3 consensus phosphorylation motif. The size of the letter is proportional to the signal for the corresponding amino acid at the indicated position. (C) In vitro kinase assays using wild-type (WT) LMTK3cat as source of enzyme activity and peptide variants with individual amino acid substitutions at different positions. Data shown are the average of two separate experiments (±SEM). (D) Top: In vitro kinase assay using recombinant HSP27 as a substrate and WT LMTK3cat or kinase-dead (KD) LMTK3 mutant (LMTK3cat-KD) as source of enzyme activity. Bottom: Time course in vitro kinase assay using recombinant HSP27 and LMTK3cat. (E) Schematic representation of SILAC proteomic experiment. Western blotting analysis of LMTK3 and FLAG-LMTK3 protein levels showing the transient overexpression of full-length pCMV6-LMTK3 (FLAG-tag) plasmid. m/z, mass/charge ratio. (F) Volcano scatter plot showing the log2 “normalized ratios” (H/L) against log10 “intensity” (H+L) for each characterized phosphorylated protein (phosphopeptide) following overexpression of WT-LMTK3 in MCF7 cells. Proteins are displayed in circles based on P values from significant B test. Red, P < 0.001; yellow, 0.001 > P < 0.01; green, 0.01 > P < 0.05; blue, P > 0.05.
