Abstract
Background.
The causal relationship between gout and renal transplant outcomes is difficult to assess due to multiple interacting covariates. This study sought to estimate the independent effect of new-onset gout on renal transplant outcomes using a methodology that accounted for these interactions.
Methods.
This study analyzed data on patients in the US Renal Data System (USRDS) who received a primary kidney transplant between 2008 and 2015. The exposure was new-onset gout, and the primary endpoint was returning to dialysis >12 months postindex date (transplant date). A marginal structural model (MSM) was fitted to determine the relative risk of new-onset gout on return to dialysis.
Results.
18 525 kidney transplant recipients in the USRDS met study eligibility. One thousand three hundred ninety-nine (7.6%) patients developed new-onset gout, and 1420 (7.7%) returned to dialysis >12 months postindex. Adjusting for baseline and time-varying confounders via the MSM showed new-onset gout was associated with a 51% increased risk of return to (RR, 1.51; 95% CI, 1.03-2.20).
Conclusions.
This finding suggests that new onset gout after kidney transplantation could be a harbinger for poor renal outcomes, and to our knowledge is the first study of kidney transplant outcomes using a technique that accounted for the dynamic relationship between renal dysfunction and gout.
1. INTRODUCTION
Hyperuricemia is common among kidney transplant recipients,1,2 has been independently associated with decreased kidney function,3-5 and has been linked to the chronic use of calcineurin inhibitors (CNI),6-10 namely cyclosporin A (CsA) and tacrolimus (TAC), which form the basis of most antirejection immunosuppressant regimens. Gout is caused by the deposition of urate crystals in tissue and is the byproduct of persistent elevation of serum uric acid levels above a soluble concentration limit of 6.8 mg/dL. These crystals can deposit in joints, soft tissues, and solid organs, including the kidneys.11-13
Gout is also a frequent comorbidity in renal transplant recipients,14 and there is preliminary evidence that patients with a history of kidney transplant are more likely to experience severe gout symptoms than patients with gout and no history of transplant.15 However, estimating the independent effect of gout on renal transplant outcomes is difficult because multiple confounders obscure a clear picture of gout’s potential impact. Glomerular filtration rate (GFR) is an established predictor of graft function that fluctuates over time.16,17 Gout exposure, which may be induced by hyperuricemia, is a suspected risk factor for graft failure.18-21 Inadequate handling of the temporal dynamic between changing GFR and gout exposure may result in biased assessments of treatment effects. Thus, inferences drawn from previous Cox proportional hazard analyses,9,22,23 which do not adjust for this dynamic, may be biased.
The objective of this analysis was to estimate the independent effect of the development of new-onset gout after kidney transplantation on graft outcomes, as assessed by the need for maintenance hemodialysis following transplantation, using a methodology that accounts for the complex temporal interactions between key covariates.
2. MATERIALS AND METHODS
2.1 Data Source and Patient Selection
This retrospective cohort study analyzed data from the US Renal Data System (USRDS) under data use agreement number 2019–2022 and was reviewed and granted institutional review board exemption under 45 CFR 46.104(d)(4). The USRDS is a national registry containing patient-level clinical and administrative claims data on end-stage renal disease (ESRD) and renal transplant populations and has been described in detail in previous research.
Initial patient selection was restricted to recipients of a primary kidney transplant (excluding multiple-organ transplants) between January 1, 2006, and December 31, 2016, which represents the earliest and latest dates for which Medicare parts A, B, and D claims were available in the USRDS. The date of kidney transplant was the “index” date that served as the reference point for evaluating patients’ histories and outcomes. To ensure adequate visibility into patients’ medical histories, eligible patients were required to have at least 24 months of continuous coverage with Medicare as their primary insurer and no history of gout in the 24 months before their index date, where gout was defined as reporting at least 1 claim with a gout diagnosis code of 274.x or M10.x, according to International Classification of Diseases, Ninth or Tenth Edition, Clinical Modification, respectively [ICD-9-CM or ICD-10-CM]. To mitigate confounding due to short-term surgical complications and acute graft rejection, patients who died, experienced graft failure, or returned to dialysis within 12 months after index date were excluded. To allow opportunity to evaluate outcomes, patients were required to have at least 12 months of Medicare coverage after index date. The combination of these criteria limited patient selection to kidney transplants occurring between January 1, 2008, and December 31, 2015, which represented a maximum postindex observation period of 9 years. As CsA-based immunosuppression regimens are independent risk factor of gout,9,10 patients with missing immunosuppression records at transplantation were excluded.
2.2 Variables of Interest
The exposure of interest was new-onset gout, defined as the presence of at least 2 postindex claims reporting ICD-9-CM or ICD-10-CM diagnosis codes 274.x or M10.x, respectively. Gout exposure was considered time-varying, with the exposure date corresponding to the first observed gout claim. Return to maintenance dialysis, a surrogate endpoint for loss of renal function, was the outcome of interest, defined as a switch in a patient’s recorded treatment modality history from “Transplant” to “Hemodialysis” or “CAPD” for continuous ambulatory peritoneal dialysis.
Baseline time-invariant confounders included kidney transplant recipient age, sex, race, body mass index (BMI), and mean estimated glomerular filtration rate (eGFR) (calculated via Chronic Kidney Disease Epidemiology Collaboration equation24) at index, and transplant donor type (living or cadaveric), age, sex, and blood-type match. Recipient history of hypertension or diabetes was evaluated over the 24 months before index and defined as the presence of at least 1 claim reporting ICD-9-CM or ICD-10-CM codes of 362.11, 401.x-405.x, 437.2, H35.0x, I10.x—I15.x, I67.4, or N26.2 for hypertension and 249.x, 250.x, 357.2, 362.0x, 366.41, or E08.x—E13.x for diabetes, respectively. Delayed graft function (defined as need for some dialysis within 1 week after transplantation) was assessed in the first week after index while mean eGFR and acute rejection status were assessed at 6 months after index. Patients were assigned to 1 of 3 transplant eras (2008–2010, 2011–2014, and 2014–2015) based on their year of transplantation.
Time-varying confounders included BMI-adjusted TAC and CsA dose, evaluated in 12-month intervals following index, postindex eGFR, and a binary indicator for whether a patient was on a urate lowering therapy (ULT) (ie, febuxostat or allopurinol).
2.3 Statistical Analysis
Descriptive analyses were performed for all baseline patient characteristics separately for patients observed/not observed to develop new-onset gout. Categorical data were described as frequencies and group differences were tested through chi-squared tests. Continuous variables were described via histograms and summary metrics (means or medians); group differences were tested through Wilcoxon rank-sum tests.
An extended Kaplan-Meier plot25 was used to graphically illustrate the cumulative probability of return to dialysis in the context of a time-varying exposure. A log-rank test was used to compare survival functions for patients with and without new-onset gout.
A marginal structural model (MSM) was fit to estimate the causal effect of new-onset gout (exposure) on patients’ return to maintenance dialysis, while controlling for both time-invariant (baseline) and time-varying confounders. The MSM was used as an alternative to a conventional Cox proportional hazards model because it has been shown to reduce the potential bias generated in the presence of a temporal relationship between the time-varying exposure and covariates.26 Specific to this study, the time-varying covariates under consideration (eGFR, ULT use and CNI dose) have been shown to affect graft outcomes27 and gout development,7,9,10 while the presence of new-onset gout predicts the subsequent covariate levels.9,28 Rather than adjusting for the time-varying covariates as regressors, the MSM used the covariates to calculate weights for an inverse-probability-of-treatment weighted estimator, which estimated the relative risk associated with new-onset gout while controlling for baseline patient and donor factors. Patients who died or lost Medicare coverage 12 months or more after index Date were censored on the date of those events; all patients remaining at the end of the study period (December 31, 2016) were also censored.
Missing time-varying covariate data was imputed via the method of multiple imputation using chained equations.29 For all analyses, results were considered statistically significant with a 2-tailed P < 0.05. All analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. RESULTS
In total, 18 525 kidney transplant recipients in the USRDS met study eligibility. Within the observation period, 1399 (7.6%) developed new-onset gout and 1420 (7.7%) returned to dialysis >12 months postindex. Figure 1 shows patient flow and exclusion criteria.
FIGURE 1.
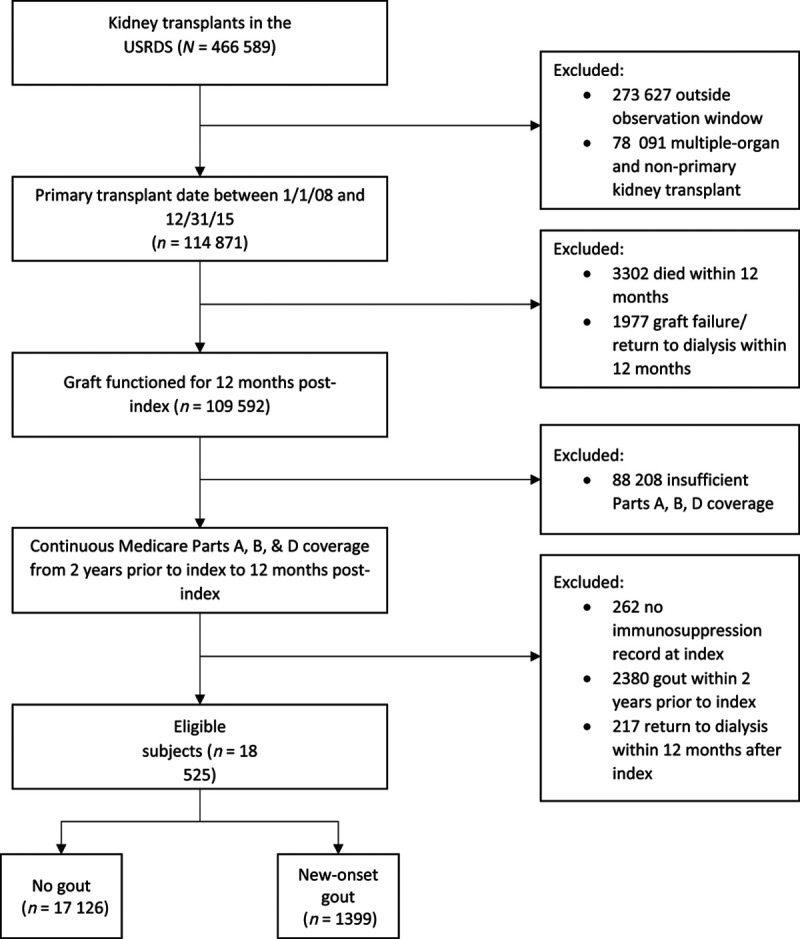
Study design flow. USRDS, US Renal Data System.
Baseline time-invariant characteristics are shown in Table 1 for new-onset gout and nongout cohorts. Recipients who developed new onset-gout were, on average, more likely to be older, male, nonwhite, and have higher BMI, as well as less likely to receive a kidney from a living donor and received a graft from an older donor (all P < 0.001, except for living donor type, P = 0.008). Patients with new-onset gout were also more likely to have longer duration on dialysis before index, history of diabetes, receive CsA, lower mean eGFR at and 6 months after index, acute rejection, and delayed graft function (all P < 0.001 except for time on dialysis, 0.009, and history of diabetes, 0.006).
TABLE 1.
Baseline time-invariant recipient and donor characteristics for no gout vs new onset gout cohorts
| Characteristics | No gout | New-onset gout | P |
|---|---|---|---|
| N (%) | 17 126 (100%) | 1399 (100%) | - |
| Mean age at index (y) | 17 126 (49.8) | 1399 (55.1) | <0.0001 |
| Sex (% female) | 7137 (42%) | 462 (33%) | <0.0001 |
| Race (% nonwhite) | 7942 (46%) | 584 (42%) | <0.0001 |
| Median BMI at index (kg/m2) | 17 126 (27.5) | 1399 (29.2) | <0.0001 |
| Mean eGFR at index (mL/min/1.73 m2) | 17 126 (37.0) | 1399 (30.1) | <0.0001 |
| Mean eGFR 6 mo postindex (mL/min/1.73 m2) | 17 126 (63.8) | 1399 (54.3) | <0.0001 |
| Median time on dialysis preindex (mo) | 17 126 (61.3) | 1399 (59.6) | 0.0098 |
| History of hypertension (% yes) | 16 520 (96%) | 1362 (97%) | 0.0791 |
| History of diabetes (% yes) | 8467 (49%) | 745 (53%) | 0.0061 |
| Mean donor age (y) | 17 126 (38.5) | 1399 (41.8) | <0.0001 |
| Donor sex (% female) | 7155 (42%) | 602 (43%) | 0.3614 |
| Donor blood type match (% yes) | 4355 (25%) | 360 (26%) | 0.8021 |
| Delayed graft function w/in 1 wk (% yes) | 4293 (25%) | 418 (30%) | <0.0001 |
| Donor type (% cadaveric) | 15 050 (88%) | 1263 (90%) | 0.0078 |
| CNI type | |||
| Tacrolimus (%) | 16 086 (94%) | 1209 (86%) | <0.0001 |
| Cyclosporine (%) | 552 (3%) | 138 (10%) | |
| Neither (%) | 488 (3%) | 52 (4%) | |
| Acute rejection 6 mo postindex | |||
| Unknown | 302 (2%) | 20 (1%) | 0.0120 |
| No (%) | 15 782 (92%) | 1267 (91%) | |
| Yes (%) | 1042 (6%) | 112 (8%) | |
| Transplant era | |||
| 2008-2010 (%) | 5956 (35%) | 677 (48%) | <0.0001 |
| 2011-2013 (%) | 6279 (37%) | 504 (36%) | |
| 2014-2015 (%) | 4891 (29%) | 218 (16%) | |
BMI, body mass index; CNI, calcineurin inhibitors; eGFR, estimated glomerular filtration rate.
Median durations of time from index to new-onset gout and from index to return to dialysis were 16.2 months (IQR, 33.4) and 32.8 months (IQR, 28.4), respectively, as shown in Figure 2A and B. In Figure 3, extended Kaplan-Meier curves25 show the cumulative unadjusted risk of return to dialysis >12 months postindex is higher in the new-onset gout cohort (log rank P < 0.001). Empirically, the curves first appear to separate around 30 months postindex, with the new-onset gout cohort appearing to display faster relative growth in risk between 30 and 40 months and 60 and 80 months.
FIGURE 2.
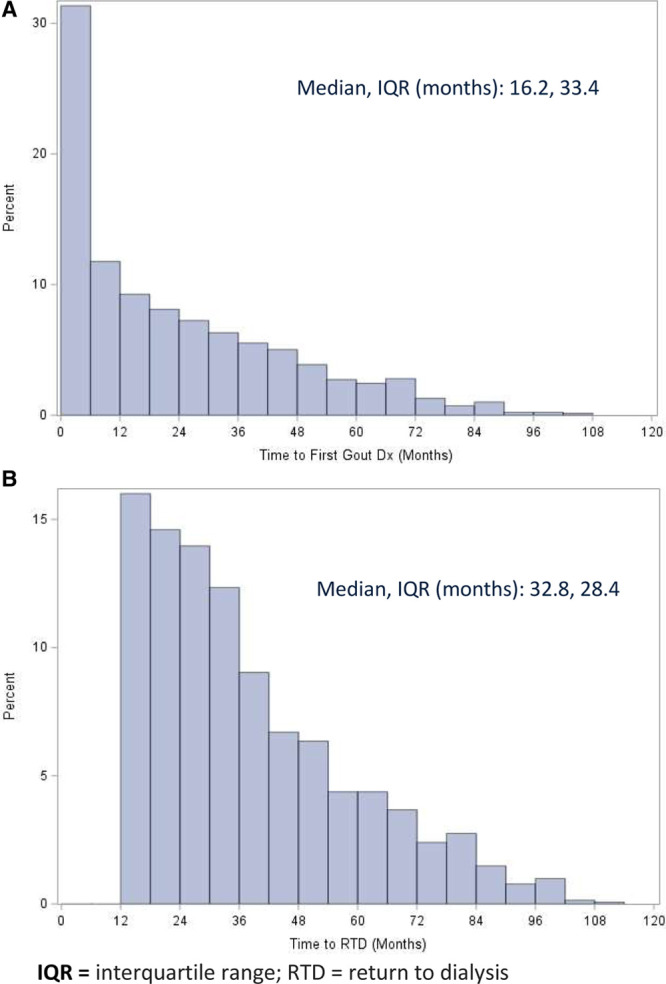
A, Distribution of duration of time from index to first diagnosis of new-onset gout. B, Distribution of duration of time from index to return to dialysis.
FIGURE 3.
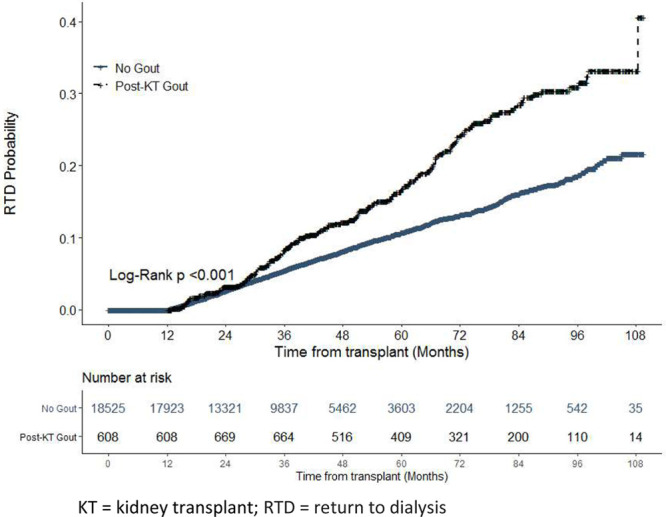
Cumulative risk of return to dialysis (unadjusted) by extended Kaplan-Meier method using new-onset gout as a time-varying exposure.
Significant risk factors for return to dialysis after adjusting for baseline time-invariant and time-varying (eGFR, ULT use, and BMI-adjusted CNI dose) confounders via the MSM are shown in Table 2. New-onset gout was associated with a 51% increased risk of return to dialysis (RR, 1.51; 95% CI, 1.03-2.20). Transplant eras 2008 to 2010 and 2011 to 2013 were also associated with significant increased risk compared with 2014 to 2016 (corresponding to 2.71, 95% CI, 2.11-3.49 and 1.79, 95% CI, 1.36-2.35, respectively). Increased risk of return to dialysis from acute rejection (1.47, 95% CI, 1.16-1.85), delayed graft function (1.34, 95% CI, 1.06-1.70), diabetes history (1.33, 95% CI, 1.05-1.67), and cadaveric donor kidney (1.27, 95% CI, 1.05-1.53) were also observed. Higher eGFR (per mL/min/1.73 m2 increase) at 6-month postindex was associated with lower risk (0.98, 95% CI, 0.97-0.98), as was older recipient age (per year of age increase) at transplantation (0.97, 95% CI, 0.96-0.97).
TABLE 2.
Association between time-varying gout and other covariates on return to dialysis 1-year after transplant assessed via marginal structural models
| Risk ratio | 95% CI | |
|---|---|---|
| New-onset gout | 1.51 | (1.03, 2.20) |
| Transplant era—2008-2010 (vs 2014-2016) | 2.71 | (2.11, 3.49) |
| Transplant era—2011-2013 (vs 2014-2016) | 1.79 | (1.36, 2.35) |
| Acute rejection episodes in first 6 mo—Yes (vs No) | 1.47 | (1.16, 1.85) |
| Delayed graft function—Yes (vs No) | 1.34 | (1.06, 1.70) |
| History of diabetes—Yes (vs No) | 1.33 | (1.05, 1.67) |
| Cadaveric donor kidney | 1.27 | (1.05, 1.53) |
| eGFR 6 mo post index—per mL/min/1.73 m2 increase | 0.98 | (0.97, 0.98) |
| Recipient age—per y of age older | 0.97 | (0.96, 0.97) |
CI, confidence interval; eGFR, estimated glomerular filtration rate.
4. DISCUSSION
In the present study, the independent effect of new-onset gout on return to maintenance hemodialysis following kidney transplantation was assessed using a methodology that simultaneously controlled for time-invariant and time-varying confounders that also affect exposure. We found that new-onset gout was independently associated with a 51% increased risk of return to dialysis (RR, 1.51; 95% CI, 1.03-2.20) >1 year after primary kidney transplantation compared with a control cohort without gout. To our knowledge, this outcome has not been observed in a well-controlled cohort study of kidney transplant recipients with gout. Although Abbott et al found an independent association between new-onset gout and greater risk of mortality (HR, 1.26; 95% CI, 1.08-1.47) and graft loss (HR, 1.22; 95% CI, 1.01-1.49) in time-dependent Cox regression analyses,9 the present study represents 3 main advancements. First, our use of a MSM provides potentially less biased estimates of the causal relationship between exposure and outcome. This approach allowed us to adjust for time-varying confounding of new-onset gout and returned to dialysis by eGFR, ULT use, and BMI-adjusted CNI dose, and thus reduced the potential bias from these time-varying dynamics. Second, Medicare Part D claims not available to Abbott et al allowed us to adjust for known confounders in ULT use and CNI dose. Finally, the risk estimates reported in the present study were observed in a maximum follow-up period of 9 years, compared with Abbott et al results based on a 3-year maximum, and thus allowed us to observe a longer-term effect of gout on graft function loss.
Our main finding on the effect of new-onset gout appear consistent with findings from a study by Kim DG et al18 which found hyperuricemia (defined as a uric acid level >7.0 mg/dL in men and >6.0 mg/dL in women) increased risk for primary endpoints of overall graft failure (HR, 2.27; 95% CI, 1.33-3.78) and death-censored graft failure (HR, 2.38; 95% CI, 1.09-4.9) after adjusting for time-varying confounding eGFR and time-varying uric acid exposure via MSMs. In contrast, using a similar adjustment approach Kim ED et al30 observed a modest protective effect from increasing uric acid levels by 10 µmol/L (0.17 mg/dL) for primary endpoints of overall graft failure (HR, 0.90; 95% CI, 0.85-0.94), death-censored graft failure (HR, 0.86; 95% CI, 0.78-0.96), and death with a functional graft (HR, 0.94; 95% CI, 0.90-0.98). One explanation for these conflicting results could be the use of different inclusion criteria. Like the present study, Kim DG excluded subjects with graft outcomes within 12 months after transplantation, whereas Kim ED applied a less restrictive exclusion criterion to subjects affected within 1 month after transplantation. We suspect the protective effect observed by Kim ED et al is an artifact of the timing of exposure and graft outcomes. In the present study, we observed the highest frequency of patients returning to dialysis in the months immediately following transplantation (data not shown), a time period associated with the greatest risk of graft loss from surgical complications and acute rejection,31,32 and thus a potential source of increased confounding due to unmeasured patient factors. It is possible that Kim ED et al observed an effect of survivor bias caused by the inclusion of this time-period.
Gout as a surrogate for prolonged hyperuricemia is the most natural hypothesis given the literature to date on hyperuricemia as an eGFR-independent risk factor for poor renal transplant outcomes,18-21 as well as preclinical evidence for serum uric acid in renal disease mechanisms including arteriolosclerosis, glomerular hypertension, glomerulosclerosis, and interstitial disease.33-36 However, hyperuricemia’s role in renal graft outcomes is far from conclusive, with recent retrospective studies observing no independent effect from elevated uric acid levels.30,37 Differences in patient selection criteria and statistical methods are notable in these studies and may contribute to the conflicting results. Nevertheless, in the time since gout was dismissed as a cause of chronic kidney disease, evidence for crystal-induced kidney injury has emerged.38-40 In the context of those and the present study’s findings, it is worth revisiting whether urate crystals, in addition to soluble uric acid, may be involved. Preliminary studies demonstrating the benefit of ULT on renal function41-44 may lead to the initiation of randomized controlled trials. If properly designed it may be worthwhile to determine whether the presence of crystal-confirmed gout potentially including urate deposition in native or transplanted kidneys, in addition to hyperuricemia, is a modifying factor on any observed treatment effect.
As expected, several of the other covariates included in the MSM had significant associations with return to dialysis. This included acute rejection, delayed graft function, deceased donor grafts, and diabetes history, all of which are well-established risk factors for graft failure,45-49 and it is reasonable that these factors would also increase risk of return to dialysis.
eGFR 6 months after transplantation was included in addition to the time-varying eGFR covariate which started at 1-year after transplantation. Our findings suggest that eGFR after renal transplantation is a risk factor for poor renal outcomes independent of a subsequent gout diagnosis, similar to findings from previous studies that early posttransplant eGFR predicts graft function independent of serum uric acid levels.50,51 Conversely, the inclusion of eGFR in the MSM, further supports the conclusion that, even if lower eGFR levels do contribute to higher risk of gout, gout also contributes a risk of return to dialysis independent of prior or concurrent renal function.
Early transplant years, captured in the current study as 3-year eras, were associated with increased risk of return to dialysis, an observation consistent with Organ Procurement and Transplantation Network reports, which show a consistent downward trend in graft failure over the timeframe in question.52 Better graft outcomes can be attributed to several clinical improvements, including decrease in median kidney donor profile index and mean kidney donor risk index demonstrating improved quality of transplanted grafts, increase in the proportion of patients receiving T-cell depleting induction agents, increase in the proportion of patients receiving TAC/mycophenolate mofetil/Steroid combination therapy as a maintenance regimen, to name a few. Additionally, some degree of increased risk is likely attributable to longer observation times in the earlier transplant era. This bias arises from a study design choice to not fix a hard-cutoff time for the endpoint of the analysis, thereby allowing the model to maximize data from earlier patients and build a more robust estimate for the time-varying variables, which was the main focus of this study. Although this design may alter the magnitude of the transplant era risk ratio, it does not preclude controlling for the aforementioned clinical factors. In fact, Kaplan-Meier curves stratified by transplant era showed the return to dialysis survival curves diverging within the first-year posttransplantation within which data were available for all included patients (Figure S1, SDC, http://links.lww.com/TXD/A296).
We found risk of return to dialysis decreased with older recipient age, consistent with previous studies in which younger age at transplantation conferred a greater adjusted risk of acute rejection,53-55 possibly due to an inherent immunologic response advantage in older recipients as observed in animal models56 or perhaps better adherence to immunosuppression medications57,58 or both.59 Another factor could be inadvertent selection bias attributed to the inclusion criteria of 24 months of Medicare coverage before transplantation. It is possible that patients younger than 65 were more likely to qualify for Medicare entitlement based on disability rather than ESRD, which may be associated with poorer overall health and, in turn, worse transplantation outcomes.
The present study has several limitations. First, we could not independently verify patients’ diagnoses with laboratory data or physician notes, and instead relied on USRDS records and billing claims. In particular, the absence of laboratory data collected overtime means we are unable to account for the potential effect from duration of elevated urate, which may confound risk. Second, as noted already, potential bias maybe inadvertently introduced by earlier transplant era. Third, to better ensure comprehensive capture of patients’ medical histories, we required eligible patients to have at least 2 years of Medicare coverage before transplantation. This requirement reduced our eligible sample considerably, and therefore may limit the generalizability of our results; for instance, by possibly oversampling higher risk recipients younger than 65 years old as noted above. Fourth, although prescription drug claims were used to identify and control for ULT use and CNI dose, we did not account the potential role of drug-induced nephrotoxicity due to concomitant diuretics or allopurinol. Finally, we were unable to account for medications paid for solely out-of-pocket or over the counter as no claim would be generated.
In conclusion, new-onset gout was independently and significantly associated with a 51% increased risk of return to dialysis 1 or more years after primary kidney transplantation compared with a control cohort without gout. To our knowledge, this is the first study of kidney transplant outcomes using a statistical technique that can account for the temporally dynamic relationship between renal dysfunction and new-onset gout. Results from this analysis may have important management implications in kidney transplant patients with new-onset gout.
ACKNOWLEDGMENTS
The authors wish to thank Sumudu Dehipawala, Bhagyashree Oak, and Jackie Karlgren for their contributions reviewing and preparing the article.
Footnotes
Published online 16 November, 2020.
J.W.L., D.Y., M.D.B., and G.M. participated in research design, writing of the article, performance of the research, and data analysis. D.Y. and Z.W. performed the data analysis. B.D.L., J.D.K., M.F.S., R.J.J., N.H., K.M.F., and H.A.S. participated in research design and writing of the article.
J.W.L., D.Y., Z.W., M.D.B., N.H., K.M.F., and H.A.S. received research funding for this work from Horizon Therapeutics. B.D.L., J.D.K., and M.F.S. are employed by and own stock in Horizon Therapeutics. R.J.J. has consulted for Horizon Therapeutics.
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
This work was supported by Horizon Therapeutics.
ORCIDS: 0000-0002-1286-0820
REFERENCES
- 1.Park JT, Kim DK, Chang TI, et al. Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrol Dial Transplant.2009; 24:3520–3525 [DOI] [PubMed] [Google Scholar]
- 2.Clive DM. Renal transplant-associated hyperuricemia and gout. J Am Soc Nephrol.2000; 11:974–979 [DOI] [PubMed] [Google Scholar]
- 3.Gibson T. Hyperuricemia, gout and the kidney. Curr Opin Rheumatol.2012; 24:127–131 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Wang F, Wang X, et al. The association between plasma uric acid and renal function decline in a Chinese population-based cohort. Nephrol Dial Transplant.2012; 27:1836–1839 [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Hu J, Song N, et al. Hyperuricemia increases the risk of acute kidney injury: a systematic review and meta-analysis. BMC Nephrol.2017; 18:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RJ, Nakagawa T, Jalal D, et al. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant.2013; 28:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HY, Rocher LL, McQuillan MA, et al. Cyclosporine-induced hyperuricemia and gout. N Engl J Med.1989; 321:287–292 [DOI] [PubMed] [Google Scholar]
- 8.Noordzij TC, Leunissen KM, Van Hooff JP. Renal handling of urate and the incidence of gouty arthritis during cyclosporine and diuretic use. Transplantation.1991; 52:64–67 [DOI] [PubMed] [Google Scholar]
- 9.Abbott KC, Kimmel PL, Dharnidharka V, et al. New-onset gout after kidney transplantation: incidence, risk factors and implications. Transplantation.2005; 80:1383–1391 [DOI] [PubMed] [Google Scholar]
- 10.Kanbay M, Akcay A, Huddam B, et al. Influence of cyclosporine and tacrolimus on serum uric acid levels in stable kidney transplant recipients. Transplant Proc.2005; 37:3119–3120 [DOI] [PubMed] [Google Scholar]
- 11.Mandell B. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med.2008; 75Suppl 5S5–S8 [DOI] [PubMed] [Google Scholar]
- 12.Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician.1999; 59:925–934 [PubMed] [Google Scholar]
- 13.Klauser AS, Halpern EJ, Strobl S, et al. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol.2019; 4:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brigham MD, Milgroom A, Lenco MO, et al. Prevalence of gout in the surviving United States solid organ transplantation population. Transplant Proc.2019; 51:3449–3455 [DOI] [PubMed] [Google Scholar]
- 15.Brigham MD, Radeck LP, Mendonca CM, et al. Gout severity in recipients of kidney transplant. Transplant Proc. 2019; 51:1816–1821 [DOI] [PubMed] [Google Scholar]
- 16.Clayton PA, Lim WH, Wong G, et al. Relationship between eGFR decline and hard outcomes after kidney transplants. J Am Soc Nephrol.2016; 27:3440–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA.2014; 311:2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DG, Choi HY, Kim HY, et al. Association between post-transplant serum uric acid levels and kidney transplantation outcomes. PLoS One.2018; 13:e0209156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han M, Lee JP, Park S, et al. Early onset hyperuricemia is a prognostic marker for kidney graft failure: propensity score matching analysis in a Korean multicenter cohort. PLoS One.2017; 12:e0176786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haririan A, Metireddy M, Cangro C, et al. Association of serum uric acid with graft survival after kidney transplantation: a time-varying analysis. Am J Transplant.2011; 11:1943–1950 [DOI] [PubMed] [Google Scholar]
- 21.Haririan A, Nogueira JM, Noguiera JM, et al. The independent association between serum uric acid and graft outcomes after kidney transplantation. Transplantation.2010; 89:573–579 [DOI] [PubMed] [Google Scholar]
- 22.Justin L, Suh M, Brigham M, et al. Sat0430 impact of gout on all-cause mortality among Medicare beneficiaries with a history of kidney transplantation: a retrospective cohort study. Ann Rheumat Dis.78Suppl 21304.1–1304 [Google Scholar]
- 23.Stack AG, Johnson ME, Blak B, et al. Gout and the risk of advanced chronic kidney disease in the UK health system: a national cohort study. BMJ Open.2019; 9:e031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med.2009; 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat.2005; 59:301–307 [Google Scholar]
- 26.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology.2000; 11:550–560 [DOI] [PubMed] [Google Scholar]
- 27.First MR. Renal function as a predictor of long-term graft survival in renal transplant patients. Nephrol Dial Transplant.2003; 18(Suppl 1):i3–i6 [DOI] [PubMed] [Google Scholar]
- 28.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009-10. PLoS One.2012; 7:e50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomized interventions, with application to an Internet-based alcohol trial. Stat Med.2011; 30:3192–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim ED, Famure O, Li Y, et al. Uric acid and the risk of graft failure in kidney transplant recipients: a re-assessment. Am J Transplant.2015; 15:482–488 [DOI] [PubMed] [Google Scholar]
- 31.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant.2009; 9:527–535 [DOI] [PubMed] [Google Scholar]
- 32.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant.2012; 12:388–399 [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Lozada LG, Tapia E, Santamaría J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int.2005; 67:237–247 [DOI] [PubMed] [Google Scholar]
- 34.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension.2001; 38:1101–1106 [DOI] [PubMed] [Google Scholar]
- 35.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol.2002; 282:F991–F997 [DOI] [PubMed] [Google Scholar]
- 36.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertens (Dallas, Tex 1979).2003; 42:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalil RS, Carpenter MA, Ivanova A, et al. Impact of hyperuricemia on long-term outcomes of kidney transplantation: analysis of the FAVORIT study. Am J Kidney Dis.2017; 70:762–769 [DOI] [PubMed] [Google Scholar]
- 38.Shimada M, Johnson RJ, May WS, Jr, et al. A novel role for uric acid in acute kidney injury associated with tumor lysis syndrome. Nephrol Dial Transplant.2009; 24:2960–2964 [DOI] [PubMed] [Google Scholar]
- 39.Bjornstad P, Maahs DM, Roncal CA, et al. Role of bicarbonate supplementation on urine uric acid crystals and diabetic tubulopathy in adults with type 1 diabetes. Diabetes Obes Metab.2018; 20:1776–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant.2003; 18:664–669 [DOI] [PubMed] [Google Scholar]
- 41.Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis.2006; 47:51–59 [DOI] [PubMed] [Google Scholar]
- 42.Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis.2015; 65:543–549 [DOI] [PubMed] [Google Scholar]
- 43.Su X, Xu B, Yan B, et al. Effects of uric acid-lowering therapy in patients with chronic kidney disease: a meta-analysis. PLoS One.2017; 12:e0187550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roughley M, Sultan AA, Clarson L, et al. Risk of chronic kidney disease in patients with gout and the impact of urate lowering therapy: a population-based cohort study. Arthritis Res Ther.2018; 20:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreso F, Serón D, Gil-Vernet S, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant.1999; 14:930–935 [DOI] [PubMed] [Google Scholar]
- 46.Butala NM, Reese PP, Doshi MD, et al. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation.2013; 95:1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation.2002; 74:1377–1381 [DOI] [PubMed] [Google Scholar]
- 48.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med.2001; 344:726–731 [DOI] [PubMed] [Google Scholar]
- 49.Lim WH, Wong G, Pilmore HL, et al. Long-term outcomes of kidney transplantation in people with type 2 diabetes: a population cohort study. Lancet Diabetes Endocrinol.2017; 5:26–33 [DOI] [PubMed] [Google Scholar]
- 50.Meier-Kriesche HU, Schold JD, Vanrenterghem Y, et al. Uric acid levels have no significant effect on renal function in adult renal transplant recipients: evidence from the symphony study. Clin J Am Soc Nephrol.2009; 4:1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong KA, Johnson DW, Campbell SB, et al. Does uric acid have a pathogenetic role in graft dysfunction and hypertension in renal transplant recipients? Transplantation.2005; 80:1565–1571 [DOI] [PubMed] [Google Scholar]
- 52.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant.2019; 19:19–123 [DOI] [PubMed] [Google Scholar]
- 53.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation.2007; 84:285–291 [DOI] [PubMed] [Google Scholar]
- 54.Mendonça HM, Dos Reis MA, de Castro de Cintra Sesso R, et al. Renal transplantation outcomes: a comparative analysis between elderly and younger recipients. Clin Transplant.2007; 21:755–760 [DOI] [PubMed] [Google Scholar]
- 55.Tullius SG, Tran H, Guleria I, et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg.2010; 252:662–674 [DOI] [PubMed] [Google Scholar]
- 56.Denecke C, Bedi DS, Ge X, et al. Prolonged graft survival in older recipient mice is determined by impaired effector T-cell but intact regulatory T-cell responses. PLoS One.2010; 5:e9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobbels F, Ruppar T, De Geest S, et al. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant.2010; 14:603–613 [DOI] [PubMed] [Google Scholar]
- 58.Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation.1998; 66:1718–1726 [DOI] [PubMed] [Google Scholar]
- 59.Pratschke J, Dragun D, Hauser IA, et al. Immunological risk assessment: the key to individualized immunosuppression after kidney transplantation. Transplant Rev (Orlando).2016; 30:77–84 [DOI] [PubMed] [Google Scholar]


