Intermittent preventive treatment in pregnancy (IPTp) with monthly sulfadoxine-pyrimethamine (SP) is recommended for malaria-endemic parts of Africa, but efficacy is compromised by resistance, and, in recent trials, dihydroartemisinin-piperaquine (DP) has shown better antimalarial protective efficacy. We utilized blood samples from a recent trial to evaluate selection by IPTp with DP or SP of Plasmodium falciparum genetic polymorphisms that alter susceptibility to these drugs. The prevalence of known genetic polymorphisms associated with altered drug susceptibility was determined in parasitemic samples, including 375 collected before IPTp drugs were administered, 125 randomly selected from those receiving SP, and 80 from those receiving DP.
KEYWORDS: malaria, Plasmodium falciparum, intermittent preventive therapy, dihydroartemisinin-piperaquine, sulfadoxine-pyrimethamine
ABSTRACT
Intermittent preventive treatment in pregnancy (IPTp) with monthly sulfadoxine-pyrimethamine (SP) is recommended for malaria-endemic parts of Africa, but efficacy is compromised by resistance, and, in recent trials, dihydroartemisinin-piperaquine (DP) has shown better antimalarial protective efficacy. We utilized blood samples from a recent trial to evaluate selection by IPTp with DP or SP of Plasmodium falciparum genetic polymorphisms that alter susceptibility to these drugs. The prevalence of known genetic polymorphisms associated with altered drug susceptibility was determined in parasitemic samples, including 375 collected before IPTp drugs were administered, 125 randomly selected from those receiving SP, and 80 from those receiving DP. For women receiving DP, the prevalence of mixed/mutant sequences was greater in samples collected during IPTp than that in samples collected prior to the intervention for PfMDR1 N86Y (20.3% versus 3.9%; P < 0.001), PfMDR1 Y184F (73.0% versus 53.0%; P < 0.001), and PfCRT K76T (46.4% versus 24.0%; P < 0.001). Considering SP, prior to IPTp, the prevalence of all 5 common antifolate mutations was over 92%, and this prevalence increased following exposure to SP, although none of these changes were statistically significant. For two additional mutations associated with high-level SP resistance, the prevalence of PfDHFR 164L (13.7% versus 4.0%; P = 0.004), but not PfDHPS 581G (1.9% versus 3.0%; P = 0.74), was greater in samples collected during IPTp compared to those collected before the intervention. Use of IPTp in Uganda selected for parasites with mutations associated with decreased susceptibility to IPTp regimens. Thus, a potential drawback of IPTp is selection of parasites with decreased drug susceptibility.
INTRODUCTION
Chemoprevention is recommended to prevent malaria in high-risk groups (1). Among chemoprevention strategies, the World Health Organization recommends intermittent preventive treatment of malaria in pregnancy (IPTp) with monthly sulfadoxine-pyrimethamine (SP) in areas of Africa with moderate to high malaria transmission (2). IPTp with SP has offered modest protective efficacy against malaria, but this efficacy has been threatened by resistance to both components of this regimen (3, 4).
The components of SP target two folate pathway enzymes, dihydrofolate reductase (PfDHFR) and dihydropteroate synthetase (PfDHPS), offering synergistic activity against Plasmodium falciparum (5). A number of well-characterized mutations decrease the activities of pyrimethamine and sulfadoxine against PfDHFR and PfDHPS, respectively. Five mutations (PfDHFR 51I, 59R, and 108N and PfDHPS 437G and 540E) have been very common in P. falciparum in Uganda and surrounding countries for many years, although 540E is uncommon in West Africa (6, 7). These mutations mediate an intermediate level of resistance to SP (5). Additional mutations, notably PfDHFR 164L and PfDHPS 581G and 613T, that mediate high-level resistance to SP have been seen in other regions. These additional mutations have been uncommon in P. falciparum from most of Africa, but studies have shown increasing prevalence in some areas, including PfDHFR 164L in southwestern Uganda (8–10) and PfDHPS 581G in parts of Tanzania (11), western Uganda (9, 10), and eastern Democratic Republic of Congo (12). In women receiving IPTp with SP, the PfDHPS 581G mutation was associated with decreased malarial preventive efficacy (13) and decreased birth weight (14) in Malawi, and the prevalence of this mutation was greater in parasites collected at delivery than those collected before the initiation of IPTp in Ghana (15). It is of interest to determine if, in Uganda, the use of monthly SP for IPTp selects for an increased prevalence of mutations that mediate resistance to this regimen.
In light of concerns regarding limited efficacy of IPTp with SP in prior studies (2) and worsening P. falciparum resistance to SP, there has been interest in alternative regimens for IPTp. Recent clinical trials in Uganda (16, 17) and Kenya (18) demonstrated that IPTp with the artemisinin-based combination therapy (ACT) dihydroartemisinin-piperaquine (DP) has superior preventive efficacy against malaria during pregnancy and against placental malaria compared to SP. When used for chemoprevention, DP benefits from the long (3- to 4-week) half-life of piperaquine, offering strong preventive efficacy with monthly dosing in children (19, 20) and pregnant women (16–18). However, the poor pharmacokinetic match between dihydroartemisinin and piperaquine engenders a risk of selection of parasites with decreased susceptibility to piperaquine. In Southeast Asia, resistance to both dihydroartemisinin and piperaquine has led to frequent failures after treatment of malaria with DP (21, 22). Importantly, the known genetic markers associated with piperaquine resistance in Southeast Asia, increased copy number of plasmepsin genes (23, 24) and novel mutations in PfCRT (25) are not prevalent in Africa (4). However, the antimalarial activity of piperaquine may also be impacted, albeit less markedly, by polymorphisms that are common in Uganda. Piperaquine is a bisquinoline related to the aminoquinolines chloroquine and amodiaquine. Mutations associated with aminoquinoline resistance, in particular, PfMDR1 86Y and PfCRT 76T, have been associated with decreased piperaquine susceptibility and selected by prior treatment with DP in some studies, although selection does not appear to be as marked as that with other aminoquinolines (26–28), and the prevalence of these mutations has decreased over time in Uganda (9, 10).
To consider selection by IPTp with DP or SP of potential drug resistance mediators in the context of changing parasite genotypes in Uganda over time, we compared the prevalence of key P. falciparum resistance markers known to be circulating in Uganda between parasites collected from pregnant women before or after the initiation of IPTp.
RESULTS
Study samples.
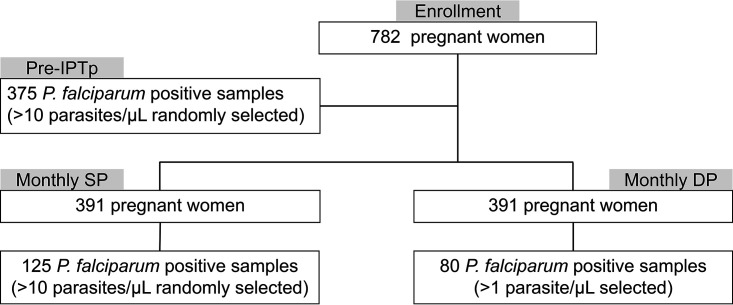
Study subjects were pregnant women in Busia District, Uganda, who were enrolled between 16 and 20 weeks of gestation in a randomized trial comparing monthly SP or DP as IPTp (17). Study subjects were all HIV uninfected and at least 16 years of age. To reach our desired sample size, we randomly selected P. falciparum-positive samples with parasitemia of at least 10 parasites/μl, both from samples collected prior to administration of the first dose of IPTp and from samples from the SP arm following the administration of IPTp with SP. We selected all samples from the DP arm following the administration of IPTp with DP with parasitemia of at least 1 parasite/μl, as this arm had fewer positives due to the greater preventive efficacy of DP (Fig. 1).
FIG 1.
Sample selection. Means of selecting samples are shown.
Selection of transporter polymorphisms.
We compared the prevalence of 4 genetic polymorphisms that have been common in Africa in P. falciparum collected from women before the onset of IPTp or during the course of monthly IPTp with DP. For PfMDR1 86Y, PfMDR1 184F, and PfCRT 76T, mutations were more prevalent in parasites collected while women received IPTp with DP than in parasites collected before the onset of IPTp or in parasites collected while women received IPTp with SP (Table 1). For the other tested polymorphism, PfMDR1 D1246Y, the prevalence of the mutation was similar in parasites collected before or during IPTp. IPTp with SP was not associated with changes in transporter polymorphisms.
TABLE 1.
Associations between exposure to IPTp and transporter gene polymorphisms
| Locus | Drug exposure | Mutation prevalence (no. mixed and mutant/total no. samples [%]) | Prevalence ratioa (95% CI) | P value | Prevalence ratiob (95% CI) | P value |
|---|---|---|---|---|---|---|
| pfmdr1N86Y | Before IPTp | 12/307 (3.9) | Reference | |||
| SP | 7/113 (6.2) | 1.58 (0.64–3.93) | 0.32 | Reference | ||
| DP | 15/74 (20.3) | 5.19 (2.53–10.6) | <0.001 | 3.27 (1.40–7.64) | 0.005 | |
| pfmdr1Y184F | Before IPTp | 158/298 (53.0) | Reference | |||
| SP | 52/116 (44.8) | 0.85 (0.67–1.06) | 0.15 | Reference | ||
| DP | 54/74 (73.0) | 1.38 (1.16–1.64) | <0.001 | 1.63 (1.27–2.08) | 0.0002 | |
| pfmdr1D1246Y | Before IPTp | 22/299 (7.4) | Reference | |||
| SP | 8/109 (7.3) | 0.99 (0.46–2.18) | 0.99 | Reference | ||
| DP | 5/74 (6.8) | 0.92 (0.36–2.35) | 0.78 | 0.92 (0.31–2.70) | 0.88 | |
| pfcrtK76T | Before IPTp | 72/300 (24.0) | Reference | |||
| SP | 25/96 (26.0) | 1.09 (0.73–1.61) | 0.68 | Reference | ||
| DP | 26/56 (46.4) | 1.93 (1.37–2.74) | <0.001 | 1.78 (1.15–2.77) | 0.01 |
Reference group is Before IPTp.
Reference group is SP.
Selection of antifolate polymorphisms.
We compared the prevalence of antifolate polymorphisms known to be prevalent in Africa in P. falciparum collected from women before the onset of IPTp or during the course of monthly IPTp with SP. For all 5 mutations that have been common in P. falciparum circulating in East Africa for many years (PfDHFR 51I, PfDHFR 59R, PfDHFR 108N, PfDHPS 437G, and PfDHPS 540E), prevalence was >92% in parasites collected before IPTp, and mutation prevalence was slightly but not significantly higher in parasites collected during IPTp with SP (Table 2). One of these mutations, PfDHPS 540E was less common in parasites from women receiving IPTp with DP than in those collected before therapy or from women receiving IPTp with SP. One additional mutation (PfDHFR 164L) associated with higher-level resistance to SP was more common in parasites collected during IPTp with SP than those collected before therapy.
TABLE 2.
Associations between exposure to IPTp and antifolate gene polymorphisms
| Locus | Drug exposure | Mutation prevalence (no. mixed and mutant/total no. samples [%]) | Prevalence ratioa (95% CI) | P value | Prevalence ratiob (95% CI) | P value |
|---|---|---|---|---|---|---|
| pfdhfrN51I | Before IPTp | 266/281 (94.7) | Reference | |||
| DP | 66/70 (94.3) | 1.00 (0.93–1.06) | 0.99 | Reference | ||
| SP | 108/113 (95.6) | 1.01 (0.96–1.06) | 0.80 | 1.01 (0.95–1.09) | 0.73 | |
| pfdhfrC59R | Before IPTp | 282/305 (92.5) | Reference | |||
| DP | 66/70 (94.3) | 1.02 (0.95–1.09) | 0.80 | Reference | ||
| SP | 116/121 (95.9) | 1.04 (0.99–1.09) | 0.28 | 1.02 (0.95–1.09) | 0.73 | |
| pfdhfrS108N | Before IPTp | 306/310 (98.7) | Reference | |||
| DP | 67/67 (100) | 1.01 (1.00–1.02) | 0.99 | Reference | ||
| SP | 119/119 (100) | 1.01 (1.00–1.03) | 0.58 | NA | NA | |
| pfdhfrI164L | Before IPTp | 9/223 (4.0) | Reference | |||
| DP | 6/66 (9.1) | 2.25 (0.83–6.10) | 0.12 | Reference | ||
| SP | 14/102 (13.7) | 3.40 (1.52–7.60) | 0.004 | 1.51 (0.61–3.73) | 0.47 | |
| pfdhpsA437G | Before IPTp | 277/281 (98.6) | Reference | |||
| DP | 61/61 (100.0) | 1.01 (1.00–1.03) | 0.99 | Reference | ||
| SP | 91/93 (97.8) | 0.99 (0.96–1.03) | 0.64 | 0.98 (0.95–1.01) | 0.52 | |
| pfdhpsK540E | Before IPTp | 302/309 (97.7) | Reference | |||
| DP | 39/58 (67.2) | 0.69 (0.57–0.82) | <0.001 | Reference | ||
| SP | 110/112 (98.2) | 1.00 (0.98–1.04) | 0.99 | 1.46 (1.22–1.75) | <0.001 | |
| pfdhpsA581G | Before IPTp | 9/303 (3.0) | Reference | |||
| DP | 0/51 (0.0) | NAc | 0.37 | Reference | ||
| SP | 2/106 (1.9) | 0.64 (0.14–2.89) | 0.74 | NA | 0.99 | |
| pfdhpsA613S | Before IPTp | 0/295 (0.0) | Reference | |||
| DP | 0/68 (0.0) | NA | NA | Reference | ||
| SP | 0/111 (0.0) | NA | NA | NA | NA |
Reference group is Before IPTp.
Reference group is SP.
NA, not applicable, as the prevalence ratio cannot be calculated.
DISCUSSION
We studied associations between the use of two regimens for IPTp and the selection of P. falciparum genetic polymorphisms associated with decreased parasite susceptibility to those regimens. SP, the established regimen for IPTp across Africa, is challenged by resistance, and parasites from women who received IPTp with SP had increased prevalence of one resistance-associated parasite mutation, PfDHFR 164L. Use of DP, an artemisinin-aminoquinoline combination under study as a new agent for IPTp, was associated with significant increases in the prevalence of mutations associated with decreased aminoquinoline susceptibility.
SP has been the standard agent for IPTp for many years. It benefits from ease of dosing (a single monthly oral dose) and established safety, but its efficacy has been limited for many years by a high prevalence of mutations in the target enzymes PfDHFR and PfDHPS that mediate resistance to its component drugs (3, 4). In this setting, the primary resistance concern is the selection of additional mutations that mediate high-level SP resistance. The use of SP was associated with the selection of one of these mutations, PfDHFR 164L. The lack of selection of 581G may have been due to the low numbers of parasites with these mutations circulating in eastern Uganda, limiting opportunities for selection. The results offer some reassurance that the use of SP will not rapidly select for parasites with high-level SP resistance. However, the prevalence of these mutations has recently been increasing in western Uganda (9, 10), suggesting that continued use of SP, in addition to the use of antifolates as antibacterials and for prophylaxis in HIV infection, may be selecting for P. falciparum highly resistant to SP.
DP is a promising alternative to SP for IPTp, offering much improved antimalarial preventive efficacy, although a change in policy to adopt DP for IPTp has been slow due to concerns about selection of parasites resistant to ACTs, potential drug-related congenital malformations or toxicity, and the unexpected result that SP offers similar protection against poor birth outcomes as does DP, possibly due to nonmalarial activities of SP (16–18). The antimalarial activity of DP may be altered by mutations in the drug transporters PfCRT and PfMDR1 that are common in Africa and mediate resistance to the related aminoquinolines chloroquine and amodiaquine, although results have been inconsistent (4). Use of DP for the treatment (29, 30) or prevention (27, 28, 31) of malaria has been associated with the selection of transporter mutations in some, but not other, studies. Considering this background, it is of interest that, in our study, use of DP was strongly associated with the selection of the two transporter mutations most clearly associated with aminoquinoline resistance, PfCRT 76T and PfMDR1 86Y, and with another mutation, PfMDR1 184F, that may stabilize the fitness of parasites with the other mutations. Thus, the use of DP in this setting selected for parasites with reduced susceptibility to amodiaquine, a component of the widely used ACT artesunate-amodiaquine, and possibly reduced susceptibility to piperaquine. Interestingly, parasites with these mutations are more sensitive than wild-type parasites to lumefantrine, a component of artemether-lumefantrine, the ACT that is the first-line antimalarial in Uganda and much of Africa.
In Southeast Asia, DP efficacy is severely challenged by resistance to both components of this regimen, associated with additional polymorphisms, specifically, kelch protein propeller domain mutations for artemisinins and plasmepsin gene amplification and/or novel PfCRT mutations for piperaquine (23–25). Due to cost constraints, we did not test for these polymorphisms in this study, but multiple other recent studies have shown their near absence in Uganda and other sites in Africa (4).
Our study had some limitations. First, due to the high protective efficacy of DP, this arm of our study had few available samples for study. However, despite a low sample size, significant associations between DP use and parasite mutations were seen. Second, due to a low number of available samples, we studied all samples in the DP arm, compared to a random set of samples, with a higher parasitemia cutoff, in the pretreatment and SP arms. The higher parasite densities in the pretreatment and SP-treated groups might have enhanced genotyping success and so affected comparisons, especially considering identification of minority genotypes. Third, a fair percentage of our assays were unsuccessful, probably due to the relatively low parasitemias present in many of our samples, which were mostly associated with asymptomatic parasitemia. Fourth, as noted above, due to cost constraints, we did not characterize the full genomes of study samples and, in particular, did not assess polymorphisms associated with DP resistance in southeast Asia, but not to date in Africa.
In conclusion, we found that P. falciparum mutations that mediate resistance to SP are common in Uganda, with modest additional selection by IPT with SP, and that use of the promising SP replacement, DP, was associated with selection of mutations that mediate resistance to aminoquinolines. Thus, the benefits of chemoprevention must be balanced with the potential drawback of the selection of drug-resistant malaria parasites.
MATERIALS AND METHODS
Source of samples for study.
We utilized P. falciparum samples from a randomized, double-blinded trial comparing IPTp with monthly SP versus monthly DP in 782 pregnant women in Busia District, Uganda (17). Briefly, women were randomized to receive either monthly DP or monthly SP beginning at 16 to 20 weeks gestation. Blood samples were obtained before the initiation of IPTp and then during monthly scheduled routine visits and whenever subjects presented with symptomatic malaria.
Identification of P. falciparum infections.
All blood samples collected from study subjects were assessed for P. falciparum by quantitative PCR (qPCR). qPCR targeting the multicopy conserved varATS gene acidic terminal sequence of P. falciparum was performed as previously described (32).
Selection of samples for genotyping.
Blood samples positive for P. falciparum by qPCR were considered for genotyping. For samples collected prior to initiation of IPTp and samples from the SP arm of the study, both of which yielded more positive samples than needed to reach our calculated sample size, genotyping was performed on a randomly chosen subset of samples that were positive by qPCR at parasitemia of at least 10 parasites/μl, as higher parasitemias provide improved genotyping yields. For the DP arm in which a smaller number of samples was P. falciparum positive, due to the improved protective efficacy of this regimen, all samples positive by qPCR at parasitemia of at least 1 parasite/μl were genotyped. Considering a sample size formula for two proportions and power (alpha) set at 0.05, the sample size for a 3:1 ratio between control and intervention arms was calculated at 375 samples before initiation of study drugs and 125 after initiation of SP. Due to a limited number of positive samples, we evaluated all 80 samples from the DP treatment arm with at least 1 parasite/μl.
Characterization of P. falciparum genetic polymorphisms.
Genomic DNA was extracted from red blood cell pellets using the Invitrogen PureLink genomic DNA minikit following the manufacturer’s instructions. P. falciparum genetic polymorphisms of interest were characterized by ligase detection reaction-fluorescent microsphere assays, as previously described (33), with minor modification to incorporate nested PCR (34).
Statistical analysis.
In our analysis, we assumed that the selective pressure of SP and DP would be the same through the course of IPTp, regardless of the number of months of therapy before sample collection. Data analysis was performed using Stata version 16. The prevalence of genetic polymorphisms of interest before and after exposure to IPTp drugs was compared using a 2-sided Fisher’s exact test. Prevalence ratios with 95% confidence intervals were reported. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by funding from the National Institutes of Health (grants AI075045, AI089674, and HD059454). J.I.N. is supported by a Fogarty International Center Emerging Global Leader Award (K43TW010365).
We thank Thomas Katairo and Philip Orishaba for assistance with data analysis, study participants for their participation, and the study staff for their assistance.
REFERENCES
- 1.World Health Organization. 2019. World malaria report 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Desai M, Hill J, Fernandes S, Walker P, Pell C, Gutman J, Kayentao K, Gonzalez R, Webster J, Greenwood B, Cot M, Ter Kuile FO. 2018. Prevention of malaria in pregnancy. Lancet Infect Dis 18:e119–e132. doi: 10.1016/S1473-3099(18)30064-1. [DOI] [PubMed] [Google Scholar]
- 3.Desai M, Gutman J, Taylor SM, Wiegand RE, Khairallah C, Kayentao K, Ouma P, Coulibaly SO, Kalilani L, Mace KE, Arinaitwe E, Mathanga DP, Doumbo O, Otieno K, Edgar D, Chaluluka E, Kamuliwo M, Ades V, Skarbinski J, Shi YP, Magnussen P, Meshnick S, Ter Kuile FO. 2016. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis 62:323–333. doi: 10.1093/cid/civ881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrad MD, Rosenthal PJ. 2019. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis 19:e338–e351. doi: 10.1016/S1473-3099(19)30261-0. [DOI] [PubMed] [Google Scholar]
- 5.Gregson A, Plowe CV. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Francis D, Nsobya SL, Talisuna A, Yeka A, Kamya MR, Machekano R, Dokomajilar C, Rosenthal PJ, Dorsey G. 2006. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis 193:978–986. doi: 10.1086/500951. [DOI] [PubMed] [Google Scholar]
- 7.Venkatesan M, Alifrangis M, Roper C, Plowe CV. 2013. Monitoring antifolate resistance in intermittent preventive therapy for malaria. Trends Parasitol 29:497–504. doi: 10.1016/j.pt.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch C, Pearce R, Pota H, Cox J, Abeku TA, Rwakimari J, Naidoo I, Tibenderana J, Roper C. 2008. Emergence of a dhfr mutation conferring high-level drug resistance in Plasmodium falciparum populations from southwest Uganda. J Infect Dis 197:1598–1604. doi: 10.1086/587845. [DOI] [PubMed] [Google Scholar]
- 9.Tumwebaze P, Tukwasibwe S, Taylor A, Conrad M, Ruhamyankaka E, Asua V, Walakira A, Nankabirwa J, Yeka A, Staedke SG, Greenhouse B, Nsobya SL, Kamya MR, Dorsey G, Rosenthal PJ. 2017. Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. J Infect Dis 215:631–635. doi: 10.1093/infdis/jiw614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asua V, Vinden J, Conrad MD, Legac J, Kigozi SP, Kamya MR, Dorsey G, Nsobya SL, Rosenthal PJ. 2018. Changing molecular markers of antimalarial drug susceptibility across Uganda. Antimicrob Agents Chemother 63:e01818-18. doi: 10.1128/AAC.01818-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, Madebe R, Mosha JF, Joho A, Mandia V, Mrema H, Mapunda E, Savael Z, Lemnge M, Mosha FW, Greenwood B, Roper C, Chandramohan D. 2009. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at codon 581. PLoS One 4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydemir O, Janko M, Hathaway NJ, Verity R, Mwandagalirwa MK, Tshefu AK, Tessema SK, Marsh PW, Tran A, Reimonn T, Ghani AC, Ghansah A, Juliano JJ, Greenhouse BR, Emch M, Meshnick SR, Bailey JA. 2018. Drug-resistance and population structure of Plasmodium falciparum across the Democratic Republic of Congo using high-throughput molecular inversion probes. J Infect Dis 218:946–955. doi: 10.1093/infdis/jiy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutman J, Kalilani L, Taylor S, Zhou Z, Wiegand RE, Thwai KL, Mwandama D, Khairallah C, Madanitsa M, Chaluluka E, Dzinjalamala F, Ali D, Mathanga DP, Skarbinski J, Shi YP, Meshnick S, ter Kuile FO. 2015. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine-pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis 211:1997–2005. doi: 10.1093/infdis/jiu836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SM, Levitt B, Freedman B, Madanitsa M, Thwai KL, Kalilani-Phiri L, Khairallah C, Mwapasa V, Ter Kuile FO, Meshnick SR. 2020. Interactions between antenatal sulfadoxine-pyrimethamine, drug-resistant Plasmodium falciparum parasites, and delivery outcomes in Malawi. J Infect Dis 222:661–669. doi: 10.1093/infdis/jiaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tornyigah B, Coppee R, Houze P, Kusi KA, Adu B, Quakyi I, Coleman N, Mama A, Deloron P, Anang AK, Clain J, Tahar R, Ofori MF, Ndam NT. 2020. Effect of drug pressure on promoting the emergence of antimalarial resistant parasites among pregnant women in Ghana. Antimicrob Agents Chemother 64:e02029-19. doi: 10.1128/AAC.02029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, Opira B, Olwoch P, Ategeka J, Nayebare P, Clark TD, Feeney ME, Charlebois ED, Rizzuto G, Muehlenbachs A, Havlir DV, Kamya MR, Dorsey G. 2016. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajubi R, Ochieng T, Kakuru A, Jagannathan P, Nakalembe M, Ruel T, Opira B, Ochokoru H, Ategeka J, Nayebare P, Clark TD, Havlir DV, Kamya MR, Dorsey G. 2019. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet 393:1428–1439. doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 18.Desai M, Gutman J, L'Lanziva A, Otieno K, Juma E, Kariuki S, Ouma P, Were V, Laserson K, Katana A, Williamson J, ter Kuile FO. 2015. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR. 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58:1404–1412. doi: 10.1093/cid/ciu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, Sam B, Dek D, Try V, Amato R, Blessborn D, Song L, Tullo GS, Fay MP, Anderson JM, Tarning J, Fairhurst RM. 2016. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, Jittamala P, Hanboonkunupakarn B, Chutasmit K, Saelow C, Runjarern R, Kaewmok W, Tripura R, Peto TJ, Yok S, Suon S, Sreng S, Mao S, Oun S, Yen S, Amaratunga C, Lek D, Huy R, Dhorda M, Chotivanich K, Ashley EA, Mukaka M, Waithira N, Cheah PY, Maude RJ, Amato R, Pearson RD, Goncalves S, Jacob CG, Hamilton WL, Fairhurst RM, Tarning J, Winterberg M, Kwiatkowski DP, Pukrittayakamee S, Hien TT, Day NP, Miotto O, White NJ, Dondorp AM. 2019. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 19:952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, Almagro-Garcia J, Neal AT, Sreng S, Suon S, Drury E, Jyothi D, Stalker J, Kwiatkowski DP, Fairhurst RM. 2017. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis 17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, Chy S, Kim S, Ke S, Kloeung N, Eam R, Khean C, Ken M, Loch K, Bouillon A, Domergue A, Ma L, Bouchier C, Leang R, Huy R, Nuel G, Barale JC, Legrand E, Ringwald P, Fidock DA, Mercereau-Puijalon O, Ariey F, Menard D. 2017. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis 17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kumpornsin K, Takala-Harrison S, Witkowski B, Fairhurst RM, Ariey F, Menard D, Fidock DA. 2018. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun 9:3314. doi: 10.1038/s41467-018-05652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumwebaze P, Conrad MD, Walakira A, LeClair N, Byaruhanga O, Nakazibwe C, Kozak B, Bloome J, Okiring J, Kakuru A, Bigira V, Kapisi J, Legac J, Gut J, Cooper RA, Kamya MR, Havlir DV, Dorsey G, Greenhouse B, Nsobya SL, Rosenthal PJ. 2015. Impact of antimalarial treatment and chemoprevention on the drug susceptibility of malaria parasites isolated from Ugandan children. Antimicrob Agents Chemother 59:3018–3030. doi: 10.1128/AAC.05141-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nankabirwa JI, Conrad MD, Legac J, Tukwasibwe S, Tumwebaze P, Wandera B, Brooker SJ, Staedke SG, Kamya MR, Nsobya SL, Dorsey G, Rosenthal PJ. 2016. Intermittent preventive treatment with dihydroartemisinin-piperaquine in Ugandan schoolchildren selects for Plasmodium falciparum transporter polymorphisms that modify drug susceptibility. Antimicrob Agents Chemother 60:5649–5654. doi: 10.1128/AAC.00920-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad MD, Mota D, Foster M, Tukwasibwe S, Legac J, Tumwebaze P, Whalen M, Kakuru A, Nayebare P, Wallender E, Havlir DV, Jagannathan P, Huang L, Aweeka F, Kamya MR, Dorsey G, Rosenthal PJ. 2017. Impact of intermittent preventive treatment during pregnancy on Plasmodium falciparum drug resistance-mediating polymorphisms in Uganda. J Infect Dis 216:1008–1017. doi: 10.1093/infdis/jix421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Some AF, Sere YY, Dokomajilar C, Zongo I, Rouamba N, Greenhouse B, Ouedraogo JB, Rosenthal PJ. 2010. Selection of known Plasmodium falciparum resistance-mediating polymorphisms by artemether-lumefantrine and amodiaquine-sulfadoxine-pyrimethamine but not dihydroartemisinin-piperaquine in Burkina Faso. Antimicrob Agents Chemother 54:1949–1954. doi: 10.1128/AAC.01413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeka A, Wallender E, Mulebeke R, Kibuuka A, Kigozi R, Bosco A, Kyambadde P, Opigo J, Kalyesubula S, Senzoga J, Vinden J, Conrad M, Rosenthal PJ. 2019. Comparative efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Ugandan children. J Infect Dis 219:1112–1120. doi: 10.1093/infdis/jiy637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Some AF, Zongo I, Compaore YD, Sakande S, Nosten F, Ouedraogo JB, Rosenthal PJ. 2014. Selection of drug resistance-mediating Plasmodium falciparum genetic polymorphisms by seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother 58:3660–3665. doi: 10.1128/AAC.02406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. 2015. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 12:e1001788. doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leclair NP, Conrad MD, Baliraine FN, Nsanzabana C, Nsobya SL, Rosenthal PJ. 2013. Optimization of a ligase detection reaction fluorescent microsphere assay for the characterization of resistance-mediating polymorphisms in African samples of Plasmodium falciparum. J Clin Microbiol 51:2564–2570. doi: 10.1128/JCM.00904-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad MD, LeClair N, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, Muhindo M, Kamya MR, Tappero JW, Greenhouse B, Dorsey G, Rosenthal PJ. 2014. Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulate drug susceptibility in Ugandan children. J Infect Dis 210:344–353. doi: 10.1093/infdis/jiu141. [DOI] [PMC free article] [PubMed] [Google Scholar]