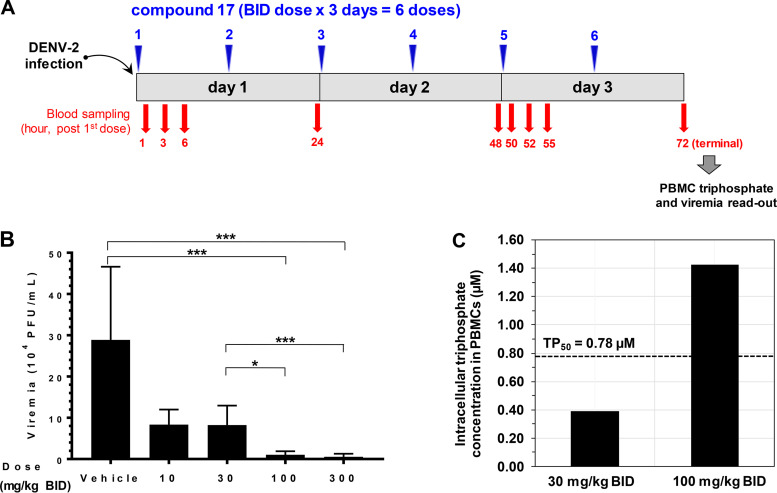
FIG 6.
Efficacy study and PBMC triphosphate analysis in the dengue viremia mouse model. (A) Dosing scheme of efficacy study in AG129 mice. Compound 17 was dosed p.o. immediately after infection at 10, 30, 100, and 300 mg/kg BID for 3 days (total doses of 20, 60, 200, and 600 mg/kg/day). Each dose group contained 6 mice. Plasma was sampled at 1, 3, 6, 24, 48, 50, 52, 55, and 72 h after the first dose for intact prodrug and nucleoside 7 analysis. A plasma sample was also taken at 72 h after the first dose (terminal sampling) for viremia readout. PBMCs were collected during the terminal time points for triphosphate analysis. (B) Compound 17 shows efficacy in AG129 mice. Viremia readout from each mouse was done on day 3 (at terminal time points) by plaque assay. Error bars represent standard deviations (n = 6 mice). Compound 17 reduced viremia by 3-, 4-, 28- and 54-fold at doses of 10, 30, 100, and 300 mg/kg/day BID, respectively. The viremia reductions at doses of 100 and 300 mg/kg BID are significant (P < 0.0001). The difference in the viremia reduction levels between the 30- and 100- or 300-mg/kg BID groups are also significant (P < 0.01 or P < 0.0001, respectively). *, P < 0.01; ***, P < 0.0001. (C) PBMC triphosphate concentration from 30- and 100-mg/kg BID groups. Blood from 6 mice was pooled at the terminal time point (72 h after the first dose) to collect PBMCs. The terminal triphosphate concentrations were 0.39 and 1.43 μM for the 30- and 100-mg/kg BID groups, respectively. The terminal triphosphate level from the 100-mg/kg BID group exceeded TP50.