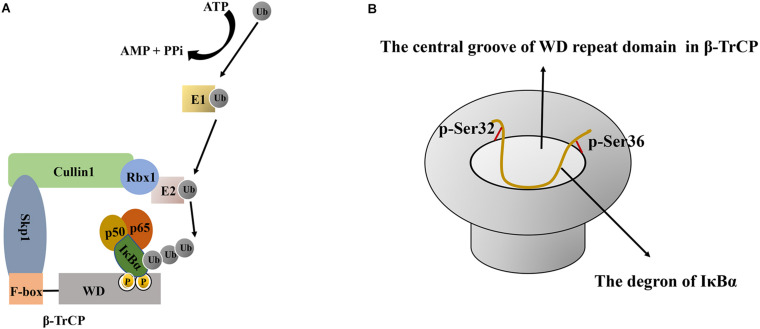
FIGURE 3.
The schematic diagram of IκBα ubiquitination and the interaction between β-TrCP and IκBα. (A) Schematic diagram of IκBα ubiquitination. First, E1- activating enzyme activates ubiquitin in an ATP-dependent manner, and then, the E1-ubiquitin intermediate transfers ubiquitin to E2 to form an E2-ubiquitin intermediate. Finally, IκBα ubiquitination requires a specific E3 ubiquitin ligase comprising Skp1, Cullin1, Rbx1, and β-TrCP. As a scaffold protein, Cullin1 can bind to the N-terminus of Skp1 and the C-terminus of Rbx1. Rbx1 binds to E2 and promotes the conversion of ubiquitin from E2 to IκBα, whereas the β-TrCP protein has two characteristic domains, namely the N-terminal F-box domain and the C-terminal WD repeat domain. The F-box domain of β-TrCP is mainly responsible for binding to Skp1, whereas the WD repeat domain is essential for IκBα recognition. (B) Schematic diagram of the interaction between the central groove of the WD repeat domain in β-TrCP and the degron of IκBα. All six residues in the IκBα degron are in contact with β-TrCP, and the phosphate groups of p-Ser32 and p-Ser36 bind at the edge of the groove. Ub, Ubiquitin; ATP, adenosine triphosphate; AMP, adenosine monophosphate; PPi, pyrophosphoric acid; IκBα, nuclear factor-kappa B inhibitor alpha; E1, E1 ubiquitin-activating enzyme; E2, E2 ubiquitin-conjugating enzyme; Skp1, S phase kinase-associated protein 1; β-TrCP, β–transducin repeat–containing protein; WD, tryptophan-aspartic acid.