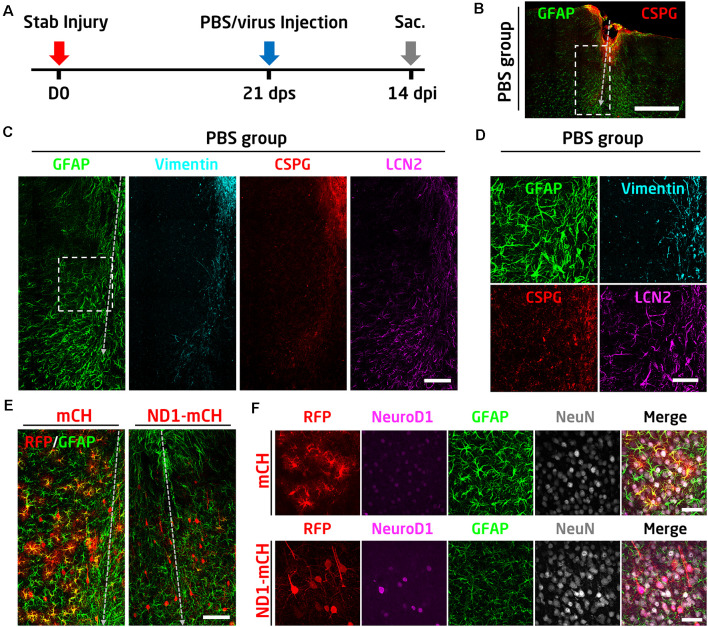
Figure 7.
NeuroD1 can convert reactive astrocytes into neurons after glial scar formation. (A) Schematic illustration of the timeline of experimental design. The viral injection was carried out at 21 days after a stab lesion when the glial scar being well established. PBS was injected as a control to assess glial scar formation. Mice were sacrificed for data analysis at 14 days after viral injection. (B) Representative image illustrating severe tissue damage in the mouse cortex at 35 days after stab injury, with PBS, injected at 21 days following stab injury as a control. Scale bar: 500 μm. (C) Enlarged images from panel B (dashed rectangle) showing densely packed hypertrophic reactive astrocytes around the stab lesion area. GFAP and LCN2 were highly expressed in the reactive astrocytes. Vimentin and CSPG were also upregulated in the injured area. Scale bar: 100 μm. (D) High magnification images from panel C (dashed square) showing the upregulation of GFAP, vimentin, CSPG, and LCN2 after stab injury. Scale bar: 50 μm. (E) Comparison of the virally infected lesion areas between mCherry control (left) and NeuroD1-treated group. Scale bar: 100 μm. (F) High magnification images showing the virally infected cells in both control and NeuroD1 groups. In the control group, mCherry-expressing cells showed reactive astrocyte morphology with a high expression level of the GFAP signal. In contrast, NeuroD1-infected cells showed neuronal morphology, and the astrocytes in the NeuroD1 group were less hypertrophic with a low level of GFAP signal compared to the control group. Scale bar: 50 μm.