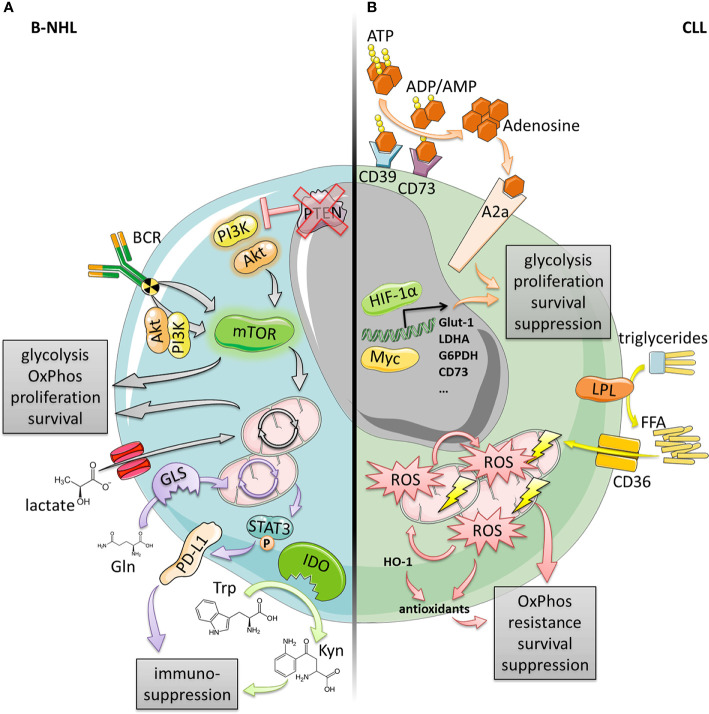
Figure 1.
Metabolic alterations in B-cell lymphomas and CLL. (A) B-NHL (DLBCL, MCL, FL) often exhibit an elevated mTOR signaling activity enabling increased glycolysis, OxPhos, proliferation and survival. This can be driven by the BCR in a PI3K/AKT-dependent or independent manner, or by genetic events such as the loss of PTEN expression resulting in constitutively active PI3K/AKT. Increased OxPhos was also found to be fueled by elevated lactate shuttling into the TCA cycle due to increased lactate importer expression. Additionally, both increased expression of IDO as well as glutaminolysis-driven PD‑L1 induction provide enhanced immune-suppression. (B) CLL cells display a high mitochondrial biomass and high levels of OxPhos generating large amounts of energy and ROS that in turn drive mitochondrial biogenesis and generation of antioxidants (at least partly by HO-1). This vicious cycle confers enhanced oxidative stress resistance, survival and suppression. OxPhos and mitochondrial biogenesis could also be driven by increased activity of LPL and CD36 consuming triglycerides and importing free fatty acids (FFA). In contrast, microenvironmental trigger (e.g., hypoxia or LN-/BM-stroma) can induce transcription factors, such as Myc or HIF-1α leading to a glycolytic switch (enabled by high metabolic flexibility), and an increase of the adenosinergic axis culminating in enhanced survival, proliferation, and suppression.