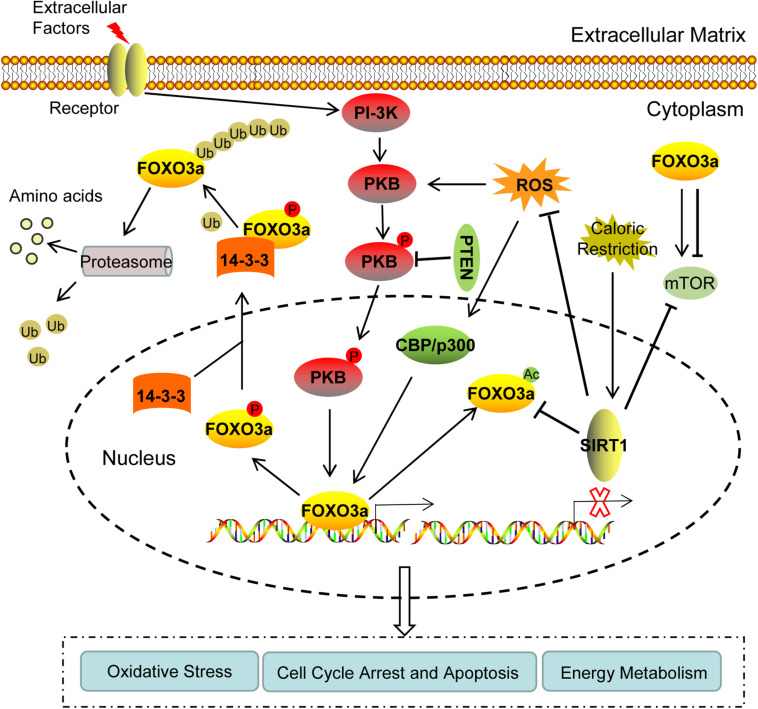
FIGURE 1.
The regulation of FOXO3a expression and its physiological role of during follicular development. (1) FOXO3a can be phosphorylated by PKB, and then transported from the nucleus to the cytoplasm by 14-3-3 molecular chaperone, accompanied by the loss of transcriptional activity. PTEN can inhibit the inactivation of FOXO3a by dephosphorylating p-PKB. (2) In the cytoplasm, the polyubiquitination of FOXO3a results in its degradation by proteasomes. (3) Acetylase CBP, p300, and other nuclear proteins can acetylate FOXO3a protein. In the nucleus, SIRT1 may activate FOXO3a by deacetylating Ac-FOXO3a. (4) Under oxidative stress, the accumulated ROS can increase post-translational modifications of FOXO3a, whereas SIRT1 can downregulate ROS. (5) FOXO3a is involved in autophagy by regulating mTOR. Caloric restriction may activate SIRT1 signaling and suppress mTOR. Collectively, the regulation of FOXO3a can mediate cell cycle arrest and apoptosis by inducing the transcription of downstream target genes, as well as by participating in oxidative stress and energy metabolism through communication with PKB, SIRT1, ROS, and mTOR, thereby affecting the activation of primordial follicles, oocyte and granulosa cell apoptosis, and regulating the growth and development of follicles. PKB, protein kinase B; PTEN, phosphatase and tensin homolog deleted on chromosome ten; SIRT1, sirtuin 1; ROS, reactive oxygen species; mTOR, mammalian target of rapamycin.