Figure 2.
Structural and Functional Assessment of Mutations
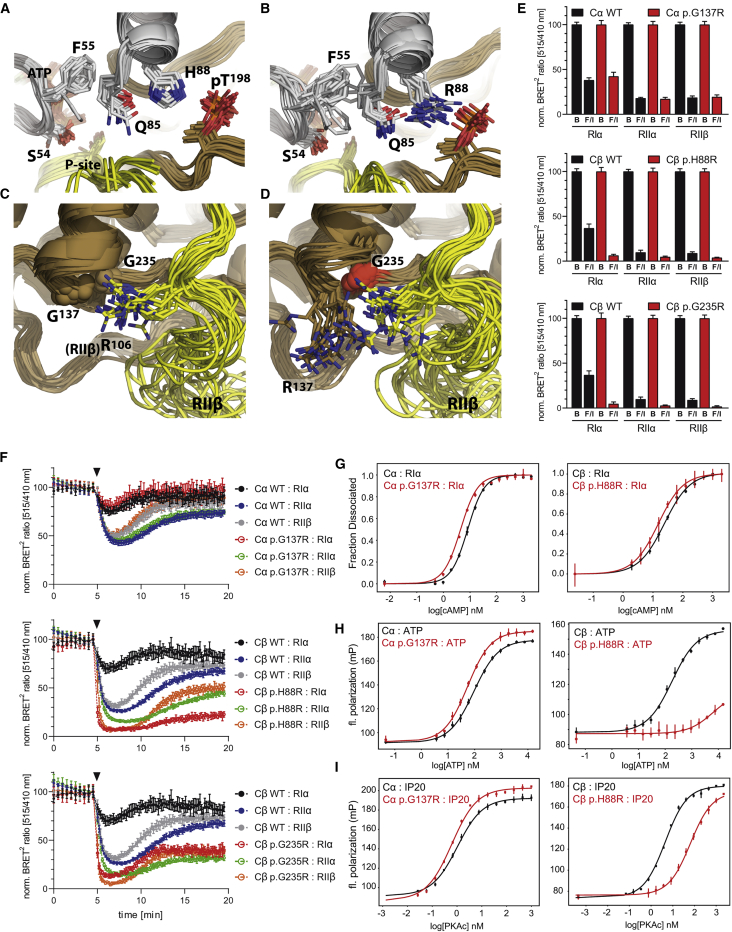
(A–D.) Ensemble model of mutations: (A) A stabilizing four-residue network that involves salt bridges between the activation loop phosphate (phospho-Thr198), the C-helix (His88 and Gln85), and hydrophobic packing of Phe55 of the G-Loop onto Gln85 is found in WT PKA. This interaction modulates the conformational dynamics of the G-Loop.
(B) The p.His88Arg (R88) mutation pulls Gln85 away from Phe55 and releases the G-Loop, likely leading to reduced synergistic binding of ATP and reduced affinity for the ATP-dependent RIα subunit. p.Ser54Leu, in the G-Loop, likely similarly affects ATP-dependent regulation by disrupting the G-Loop dynamics.
(C) In WT PKA, a pocket is formed by the D and F helices, and that pocket is accessed in an RIIβ-specific manner by Arg106 in the inhibitor segment (PDB:3TNP) and by the tethering helix in PKI(5-24).
(D) p.Gly137Arg and p.Gly235Arg disturb this RIIβ-specific interaction. This pocket is also at the RIα cAMP-binding domain-B interface (PDB:6NO7) and at the interface with the tethering helix in PKI (Figure S4B–S4C).
(E) In BRET2 experiments, Cα-p.Gly137Arg shows comparable dissociation to that of Cα-WT upon full cAMP-stimulation by Forskolin/IBMX (F/I) for RIα-, RIIα, and RIIβ-holoenzymes, whereas Cβ-p.His88Arg- and Cβ-p.Gly235Arg-holoenzymes fully dissociate. BRET2 data from unstimulated cells treated with buffer only are designated by the letter B. Normalized data are shown as means ± SD of three independent experiments with n = 6 replicates each (total n = 18).
(F) Kinetic BRET2 analyses demonstrate full dissociation upon addition of the physiological β-adrenergic agonist isoproterenol (100 nM, triangle) for Cβ-p.His88Arg and Cβ-p.Gly235Arg and identical behavior of Cα-WT and Cα-p.Gly137Arg. Data shown are means ± SD of n = 6 replicates showing one of three (two for RIIα) independent experiments. For expression levels of GFP-C-subunits used in BRET, see Figure S5B.
(G) FPA analysis: RIα holoenzyme activation by cAMP shows increased sensitivity compared to WT with both Cα-p.Gly137Arg and Cβ-p.His88Arg (n = 3).
(H–I) FPA: Synergistic binding of ATP (H) with PKI 5-24 (IP20) peptide (I) to the C-subunit shows slightly increased binding affinity compared to WT with Cα-p.Gly137Arg and strongly decreased cooperativity with Cβ-p.His88Arg (n = 3). To illustrate differences in total binding, raw fluorescence polarization is expressed as millipolarization units (mP), and otherwise mP has been converted to fraction dissociated. Graphs show the mean ± SD.