Figure 3.
PRKACA and PRKACB Mutations Impair Hh Signaling in NIH 3T3
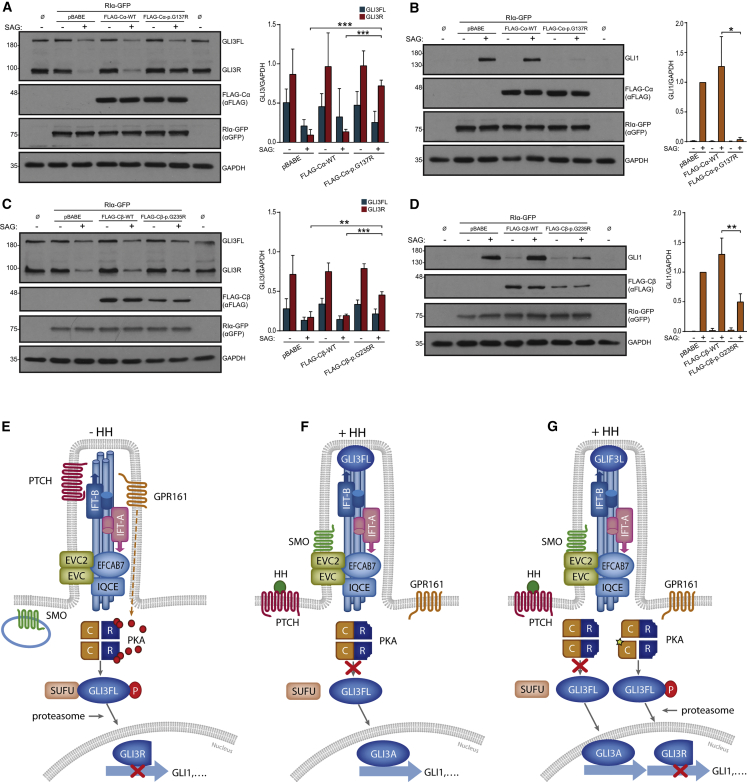
(A–D) Analysis of GLI3 and GLI1 protein levels in NIH 3T3 co-infected with human FLAG-Cα-WT or FLAG-Cα-p.Gly137Arg and RIα-GFP retroviral vectors (A–B) or alternatively with FLAG-Cβ-WT or FLAG-Cβ-p.Gly235Arg and RIα-GFP retroviruses (C–D), exposed to SAG (+) or its vehicle DMSO (-). Non-infected cells are indicated with Ø. Expression levels of FLAG-C and RIα-GFP are shown in the underneath panels. After incubation with SAG, Cα-p.Gly137Arg and Cβ-p.Gly235Arg retrotransduced cells showed increased GLI3R protein levels and reduced expression of GLI1 compared to cells retrotransduced with pBABE (empty vector) or with FLAG-Cα-WT or FLAG-Cβ-WT. Representative immunoblots are on the left and histograms show densitometric quantification of the levels of GLI3R and GLI3FL referred to GAPDH in (A) and (C), or GLI1/GAPDH levels normalized to the value of SAG-pBABE cells in (B) and (D). Data are expressed as mean ± SD from three experiments corresponding to three independent retroviral infections (n = 3). ∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001. Student’s t-test.
(E–G) Model of action of PKA-Cα/β mutations. (E) In the absence of signal, PTCH (the receptor of Hh ligands [HH]) is in the cilium and represses SMO. GPR161 is also located in the cilium membrane and negatively regulates Hh signaling by promoting adenylyl-cyclase-dependent cAMP synthesis (red spheres). Consequently, PKA holoenzymes are active and their C-subunits (C) are free from R-subunits (R) to phosphorylate GLI3FL, which is bound to the inhibitory protein SUFU. Phosphorylated GLI3FL undergoes C-terminal proteolytic processing by the proteasome and is transformed into GLI3R, leading to reduced expression of Hh targets such as GLI1. (F) The interaction of Hh ligands with PTCH disables this protein to continue repressing SMO and PTCH-HH complexes exit from cilia. De-repressed SMO accumulates into the cilium and interacts with the EvC ciliary complex, which is retained at the base of this organelle through binding of the C-terminal of EVC2 to the EFCAB7-IQCE complex.19 In this manner, SMO signaling is enriched at the EvC region. SMO and the EvC proteins promote GLI3FL-SUFU dissociation and stimulate the recruitment of GLI3 to cilia tips.20, 21, 22 Active SMO additionally causes GPR161 to abandon the cilium, and this, in combination with other not fully understood SMO-mediated mechanisms, results in decreased levels of cAMP and the inactivation of PKA.4,23 Accordingly, GLI3FL phosphorylation is stopped and the production of GLI3R discontinued while GLI3FL is converted into a functional transcriptional activator (GLI3A). (G) The same situation as in (F), but in an individual with a PRKACA or PRKACB mutation. Due to higher cAMP sensitivity of the mutant PKA holoenzymes, the mutant PKA C-subunits (star) remain active following downregulation of cAMP levels associated with the activation of the Hh pathway, thus leading to abnormally increased levels of GLI3R and reduced Hh pathway activity. Affected individuals are expected to have holoenzymes containing two normal or two mutant C-subunits and holoenzymes composed of one normal and one mutant C-subunit. Intraflagellar transport protein complexes (IFT-A and IFT-B) which are also involved in Hh signaling are indicated in (E–G).