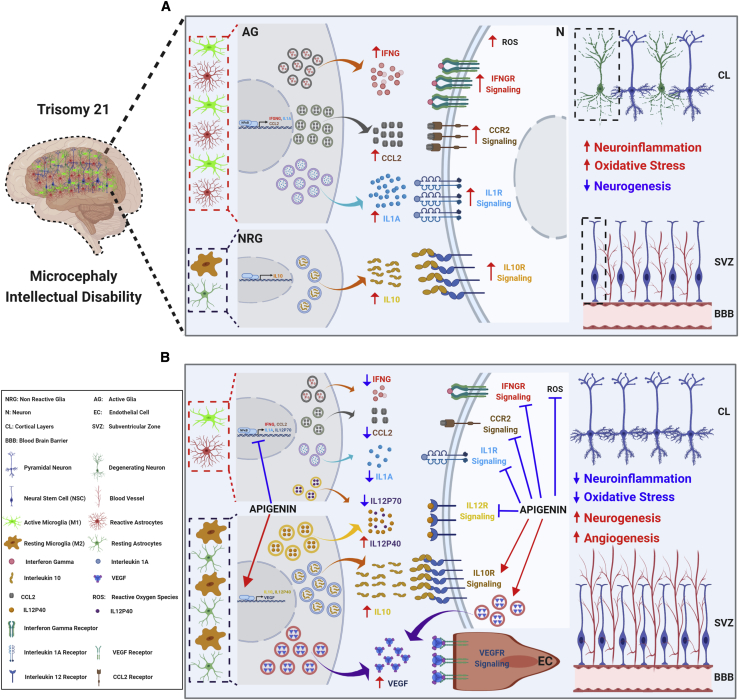
Figure 6.
Proposed Mechanisms of Action of Apigenin in the Brains of People with Trisomy 21
(A) Three copies of chromosome 21 are associated with increased oxidative stress, neuroinflammation, and reduced neurogenesis. Combining gene and protein expression data from human T21 amniocytes and embryonic forebrain of the Ts1Cje mouse model, we propose that the increased production of reactive oxygen species (ROSs) and pro-inflammatory cytokines (e.g., IFNG, IL1A, and CCL2) by reactive astrocytes and active microglia as well as cell-cycle delays in neural stem cells (NSCs) result in reduced neurogenesis and a lower number of mature neurons in the brain, which ultimately leads to microcephaly and intellectual disability.
(B) Apigenin treatment has pleiotropic actions by promoting anti-inflammatory IL10/IL10R signaling, reducing neuroinflammation through inhibition of NFκB, IFNG/IFNGR, CCL2/CCR2, IL1A/IL1AR, and IL12R signaling, promoting the expression of proneural genes in NSCs, and increasing angiogenesis through the activation of VEGF signaling. We propose that apigenin’s anti-inflammatory effects are triggered via blocking the activation of astrocytes and promoting the M2 (anti-inflammatory and neuroprotective) microglia phenotype over the M1 (pro-inflammatory and neurotoxic) phenotype.