Figure 5.
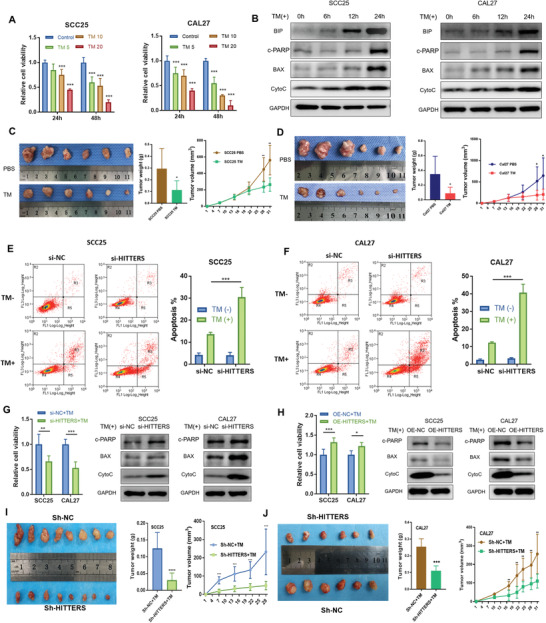
HITTERS attenuates ER stress induced apoptosis. A) Cell viability (measured by CCK8) of SCC25 and CAL27 were significantly suppressed by TM (5, 10, and 20 µg mL−1 for 24 and 48 h) in a dose‐ and time‐dependent manner. One‐way ANOVA and Dunnett t‐test were used for each time point. B) Western blot showed the ER stress marker BIP and apoptosis marker of SCC25 and CAL27 cells were significantly upregulated by TM (10 µg mL−1) in a time‐dependent manner. c‐PARP, cleaved PARP. C,D) Intraperitoneally injection of TM twice a week significantly suppressed tumor volume and tumor weight in C) SCC25 and D) CAL27 subcutaneous xenograft model. E,F) Under non‐ER stress condition, HITTERS would not affect apoptosis; however, depletion of HITTERS in TM (10 µg mL−1, 24 h) treated E) SCC25 and F) CAL27 cells significantly promoted apoptosis. Apoptosis was measured by Annexin‐V/PI double staining and flow cytometry. Proportion of R3 + R5 is considered apoptosis. Cells were transfected with siRNA for 48 h and then treated with TM (10 µg mL−1, 24 h). Two‐way ANOVA and Sidak's multiple comparisons test were used. G) Knocking‐down HITTERS significantly suppressed cell viability and promoted apoptosis marker expression; H) whereas overexpressing HITTERS obtained the opposite effect. Cells were transfected with siRNA for 48 h and then treated with TM (10 µg mL−1, 24 h). Cell viability differences were measured by CCK8 using Student t‐test. Stably knockdown of HITTERS causing I) SCC25 and J) CAL27 more sensitive to ER stress in vivo, reflected by a significantly reduction in tumor volume and tumor weight in subcutaneous xenograft model. All BALB/c nude mice were intraperitoneally injected with TM, twice a week, after tumor bearing. Student t‐test was used. Note: *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001.
