Scheme 1. Synthesis Scheme of 5 (UK101-OH) and 9 (Cfz-OH)a.
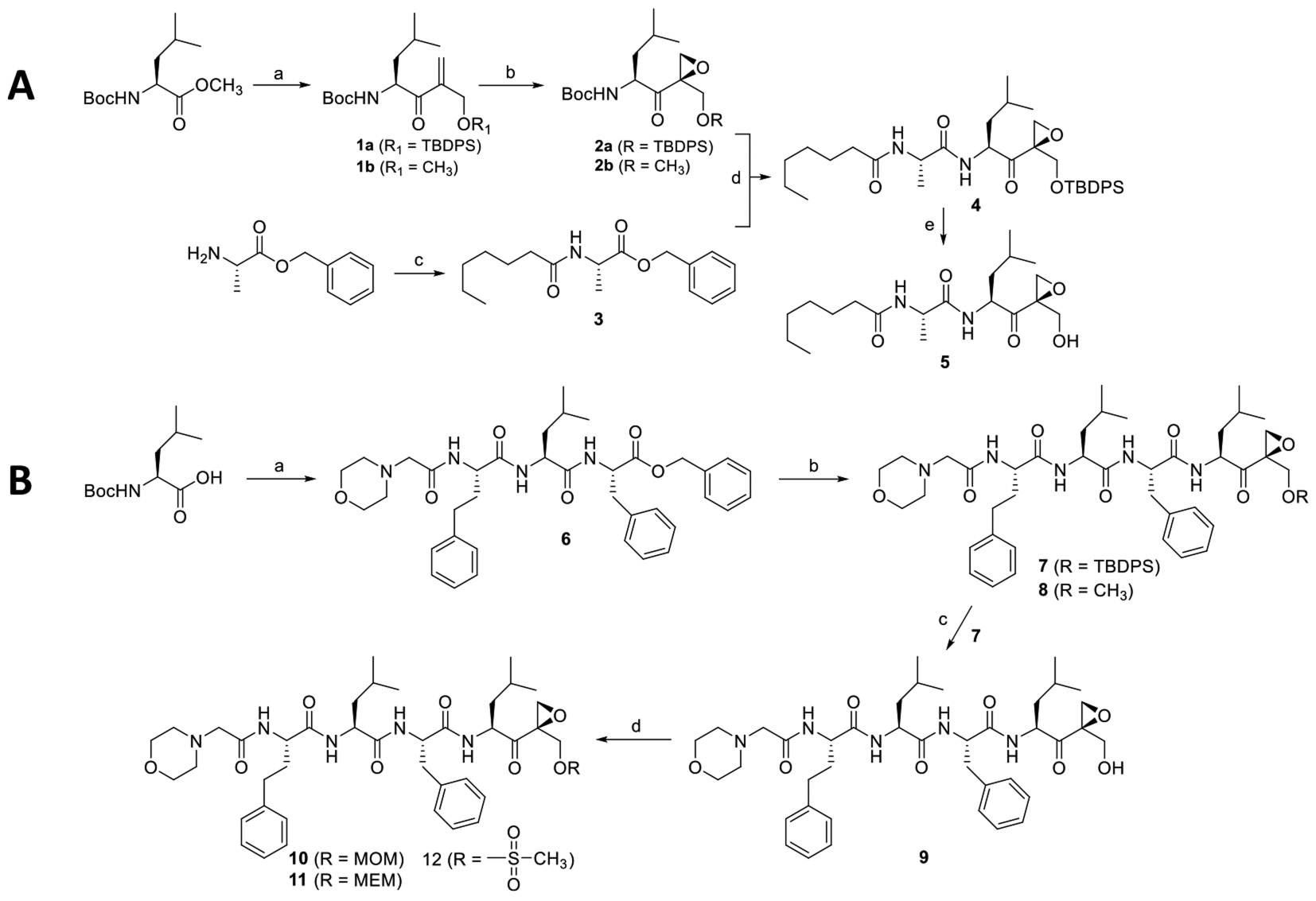
aReagents and conditions. (A) (a) (i) CH3PO(OCH3)2, n-BuLi, THF, −78 °C, 2 h, Boc-serine methylester, THF, −78 °C, 3 h, 55%; (ii) Aqueous solution of formaldehyde, K2CO3, H2O, rt, 4 h, 59%; (iii) TBDMS-Cl, imidazole, DCM, rt, 30 min, 71% or iodomethane, Ag2O, MeCN, rt, 24 h, 51%; (b) benzonitrile, H2O2, DIEA, MeOH, 0 °C, 3 h, 35–41%; (c) heptanoic acid, HBTU, HOBt, DIEA, DCM, rt, 18 h, 82%; (d) (i) H2, Pd/C, methanol, 1 h, (ii) Boc-deprotected 2a, HBTU, HOBt, DIEA, DCM, rt, 18 h, 91%; (e) TBAF, DCM, 2 h, 70%. (B) (a) (i) Phenylalanine benzyl ester hydrochloride, HBTU, HOBt, DIEA, DCM, rt, 18 h, 81%; (ii) TFA, DCM, rt, 1 h then evaporation and drying, Boc-homoPhe-OH, HBTU, HOBt, DIEA, DCM, rt, 18 h, 79%; (iii) TFA, DCM, rt, 1 h then evaporate and dried, morpholin-4-yl-acetic acid hydrochloride, HBTU, HOBt, DIEA, DCM, rt, 18 h, 65%; (b) (i) H2/Pd, C, methanol, 1 h; (ii) 2a or 2b, HBTU, HOBt, DIEA, DCM, rt, 18 h, 45% and 48%, respectively; (c) TBAF, DCM, 2 h, 64%; (d) MOM-Cl or MEM-Cl or methanesulfonyl chloride, DIEA, DCM, overnight, 60–64%.
