Figure 4.
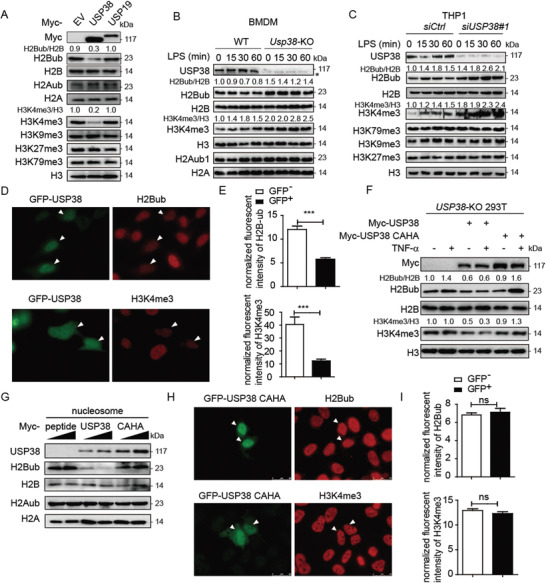
USP38 specifically reduces monoubiquitination of H2B and trimethylation of H3K4. A) Immunoblot analysis of extracts of HEK293T cells transfected with Myc‐empty vector (EV), Myc‐tagged USP38, or Myc‐USP19 with the indicated antibodies. The protein levels of H2Bub/H2B and H3K4me3/H3 were quantified by ImageJ software (NIH). B,C) Immunoblot analysis of H2Bub/H2B and H3K4me3/H3 in wild‐type (WT) and Usp38‐knockout (KO) BMDMs or control and USP38‐silenced THP1 cells with LPS treatment for the indicated time points. D,E) Immunofluorescence analysis of H2BK120ub (H2Bub) and H3K4me3 with GFP‐USP38 in HeLa cells. The relative intensity of fluorescence signals of H2Bub and H3K4me3 in GFP‐USP38 transfected cells (GFP+) versus control cells (GFP−) in the same image frame was analyzed by using ImageJ software (NIH). F) Immunoblot analysis of H2Bub/H2B and H3K4me3/H3 in USP38‐KO 293T cells with Myc‐vector, Myc‐USP38, or Myc‐USP38 CAHA overexpression under TNFα treatment. G) In vitro deubiquitylation assay of H2B by incubating purified nucleosomes with increasing amounts of purified Myc‐USP38, Myc‐USP38 CAHA mutant, or Myc‐peptide. H,I) Immunofluorescence analysis of H2Bub and H3K4me3 with the GFP‐USP38‐CAHA mutant in HeLa cells. The experimental setting is similar to that in (D) and (E). Data in (A)–(D) and (F)–(H) are representative of three independent biological experiments. Data in (E) and (I) are presented as the means ± SEM of at least three independent experiments with 50 cells/experiment. ***p < 0.001, ns, no significant difference, versus the control cells (Student's t‐test).
