Abstract
BACKGROUND
Clinical presentation and risk factors of death in COVID‐19 in oldest adults have not been well characterized.
OBJECTIVES
To describe clinical features and outcome of COVID‐19 in patients older than 85 years and study risk factors for mortality.
DESIGN
Prospective cohort.
PARTICIPANTS AND SETTING
Patients aged 85 years and older, admitted in noncritical care units at the University Hospital Lariboisière Fernand‐Widal (Paris, France) for confirmed severe acute respiratory syndrome coronavirus 2 infection were included and followed up for 21 days.
MEASUREMENTS
Clinical and laboratory findings were collected. Cox survival analysis was performed to explore factors associated with death.
RESULTS
From March 14 to April 11, 2020, 76 patients (median age = 90 (86–92) years; women = 55.3%) were admitted for confirmed COVID‐19. Of the patients, 64.5% presented with three or more comorbidities. Most common symptoms were asthenia (76.3%), fever (75.0%) and confusion and delirium (71.1%). An initial fall was reported in 25.0% of cases, and digestive symptoms were reported in 22.4% of cases. COVID‐19 was severe in 51.3% of cases, moderate in 32.9%, and mild in 15.8%. Complications included acute respiratory syndrome (28.9%), cardiac decompensation (14.5%), and hypotensive shock (9.0%). Fatality at 21 days was 28.9%, after a median course of disease of 13 (8–17) days. Males were overrepresented in nonsurvivors (68.2%). In survivors, median length of stay was 12 (9–19.5) days. Independent predictive factors of death were C‐reactive protein level at admission and lymphocyte count at nadir.
CONCLUSION
Specific clinical features, multiorgan injury, and high case fatality rate are observed in older adults with COVID‐19. However, rapid diagnosis, appropriate care, and monitoring seem to improve prognosis.
Keywords: older adults, SARS‐CoV‐2, COVID‐19, clinical research, mortality, risk factors
1. INTRODUCTION
The oldest‐old (people aged ≥85 years) population usually has a different burden of disease incidence than the general population, including high incidence and more severe symptoms requiring specific explorations and care. 1 Although their proportion is increasing dramatically worldwide, little is known about the specific features of COVID‐19 in this population of older adults.
A novel coronavirus, designated as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was identified as the cause of COVID‐19 in Wuhan, China, at the end of 2019. 2 It has spread rapidly to the rest of the world, with more than 27 million cases worldwide reported by the World Health Organization as of September 4, 2020. The clinical spectrum of SARS‐CoV‐2 pneumonia ranges from mild to critical cases. 3 Although age and comorbidities are considered to be the main risk factors for severe form and death in patients with COVID‐19, 4 clinical description in oldest‐old patients is still underexplored. So far, COVID‐19 is limited in descriptive report of epidemiological findings, clinical presentation, and clinical outcomes of patients older than 65 years. 5 , 6 , 7
We report a prospective cohort of patients, aged 85 years and older, sequentially admitted for confirmed SARS‐CoV‐2 in an academic hospital in Paris, France.
2. METHODS
We performed a monocentric prospective study at Lariboisière‐Fernand Widal Hospital, Assistance Publique–Hôpitaux de Paris, Université de Paris. All consecutive patients, aged 85 years and older, admitted with confirmed SARS‐CoV‐2 infection by positive result on polymerase chain reaction testing of a nasopharyngeal sample, were included, from March 14, 2020, to April 9, 2020. Follow‐up length was 21 days, starting from the day of the positive test. Period of follow‐up was decided on reported median delay of evolution to acute respiratory distress syndrome (ARDS) in the literature, 4 , 8 duration of disease to death, 8 , 9 and delay of viral shedding in survivors 4 to assess direct mortality of infection. None of the patients was considered a candidate for intensive care unit after a collegial discussion including at least two specialist physicians of different specialty and the palliative care team. All patients were administrated maximal care in the department in which they were admitted. If decided, palliative care and suspension of active medication (antibiotherapy and oxygen therapy) was decided in association with the palliative care team. Patients, caregivers, and/or surrogates were informed and associated to therapeutic decisions.
Data collected included patient demographic information, comorbidities, and usual medication. Frailty was evaluated using the Clinical Frailty Score. 10 Cardioneurovascular diseases included coronary artery disease, history of stroke, and peripheral arterial disease. Nutritional status was assessed at admission by the physician in charge based on body mass index (BMI), albumin levels, and prealbumin.
Initial laboratory testing was defined as test results obtained within 24 hours of the SARS‐CoV‐2 testing. Lymphocyte absolute number was nadir count. Lymphopenia was defined by an absolute count under 1.5 G/L. Thrombocytopenia was defined by an absolute count of platelets under 150 Giga/L. Acute kidney injury was diagnosed according to the recommendations. 11 Elevated troponin was defined as a result superior to upper limit of normal for the individual references ranges, as troponin I, troponin T, and high‐sensitivity troponin T were equally used in this series. Blood oxygen desaturation was defined as a loss of 3% or more or as saturation under 93%. Cardiac decompensation was defined as the association of clinical symptoms (dyspnea, orthopnea, and edema) and elevated brain natriuretic peptide (BNP). The severity of COVID‐19, ARDS, and shock was defined according to the recommendations of World Health Organization for COVID‐19. 12
Treatment and outcome were reported. The primary outcome was the 21‐day fatality on admission. Incidence of SARS‐CoV‐2–related ARDS and complications was also reported. For survivors, length of stay and discharge were studied.
Ethical approval and consent to participate: For this observational study using anonymized data, the required approval from the Commission Nationale Informatique et Liberté to collect anonymized data was obtained.
2.1. Statistical Analysis
Statistical analysis was performed using SPSS Statistics software, version 26 (IBM Corporation). Nominal variables were presented as number and percentage, whereas continuous variables were presented as median and interquartile range (IQR). Distribution of the continuous variables was performed by using the Kolmogorov‐Smirnov test. Independent‐sample t‐test was applied to analyze normally distributed data, whereas Mann‐Whitney and Kruskal‐Wallis tests were applied to analyze nonnormally distributed data. The chi‐square test was applied to examine categorical data. Clinical and laboratory variables, which differed significantly under comparative statistics between survivor and nonsurvivor groups, were included in a Cox proportional hazards regression model to identify prognostic factors of mortality. We excluded variables from the survival analysis when the number of events was too small to calculate hazard ratios (HRs) or if the number of missing data was too high. Validity of the model was checked using likelihood ratio test and Wald test. In sensitivity analysis, we reran our Cox model after exclusion of already hospitalized subjects to avoid a potential selection bias. For all analyses and comparisons, a P value of .05 or less was overall considered statistically significant.
3. RESULTS
3.1. General Characteristics
Population characteristics are described in Table 1. From March 14 to April 11, 2020, 76 patients older than 84 years were included. Median age was 90 (IQR, 86–92) years, ranging from 85 to 99 years (mean age = 90.0 ± 3.9 years). Forty‐two included patients (55.3%) were women. Twenty‐four patients (31.6%) were living in a long‐term care facility. Seventeen patients (22.4%) were already hospitalized at the time of diagnosis. Median Clinical Frailty Score was 6 (IQR, 5–7), corresponding to a “moderately frail” state (loss of autonomy for outside activities and assistance inside). Seventy‐four patients (97.4%) presented with comorbidities, among which 49 (64.5%) had three or more. The most common comorbidities were arterial hypertension in 62 cases (81.6%), cognitive impairment in 48 cases (63.2%), and cardiac insufficiency in 24 cases (31.6%). Compared with the 54 survivors, the 22 nonsurvivors were predominantly men (15 (68.2%) vs 7 (31.8%) in survivors; P = .011). In regard to comorbidities, only cardioneurovascular disease frequency differed between both groups (62.8% in nonsurvivors vs 37.0% in survivors; P = .013). Treatment by beta‐blockers and angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists did not differ between groups (respectively 34.2% and 25.0% in the survivor group; 36.4% and 27.3% in the nonsurvivor group; P > .05).
Table 1.
Clinical Characteristics of Patients
| Characteristic | Total (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
|---|---|---|---|---|
| Age, y | 90 (86–92) | 90 (86–92) | 89.50 (86–95) | .831 |
| Male sex | 34 (44.7) | 19 (35.2) | 15 (68.2) | .011 |
| BMI, kg/m2 | 22.6 (19.6–25.4) | 22.6 (19.5–24.8) | 23.4 (20.9–26.12) | .394 |
| Malnutrition | 29 (38.2) | 23 (42.6) | 6 (27.3) | .298 |
| Obesity (BMI > 30 kg/m2) | 2 (2.6) | 1 (1.9) | 1 (4.5) | .498 |
| Clinical Frailty Score | 6 (5–7) | 6 (5.7–7) | 6 (5–7) | .111 |
| Patient origin | .130 | |||
| Home | 35 (46.0) | 21 (38.9) | 14 (63.6) | |
| Long‐term care facility | 24 (31.6) | 20 (37.0) | 4 (18.2) | |
| Hospital | 17 (22.4) | 13 (24.1) | 4 (18.2) | |
| Comorbidities | .432 | |||
| 0 | 2 (2.6) | 1 (1.9) | 1 (4.6) | |
| 1–2 | 25 (32.9) | 20 (37.0) | 5 (22.7) | |
| >2 | 49 (64.5) | 33 (61.1) | 16 (72.7) | |
| Medical history | ||||
| Cardioneurovascular diseases | 35 (46.1) | 20 (37.0) | 15 (68.2) | .013 |
| Arterial hypertension | 62 (81.6) | 45 (83.3) | 17 (77.3) | .531 |
| Coronary artery disease | 15 (19.7) | 8 (14.8) | 7 (31.8) | .116 |
| Cardiac insufficiency | 24 (31.6) | 19 (35.2) | 5 (22.7) | .416 |
| History of thrombosis | 9 (11.8) | 9 (16.7) | 0 (0.0) | .052 |
| COPD | 6 (7.9) | 4 (7.4) | 2 (9.1) | 1.000 |
| Respiratory insufficiency | 5 (6.6) | 4 (7.4) | 1 (4.5) | 1.000 |
| History of stroke | 22 (28.9) | 17 (31.5) | 5 (22.7) | .580 |
| Cognitive impairment | 48 (63.2) | 38 (70.4) | 10 (45.5) | .055 |
| Neurodegenerative disease | 13 (17.1) | 7 (13.0) | 6 (27.3) | .180 |
| Chronic kidney disease | 20 (26.3) | 11 (20.4) | 9 (40.9) | 1.000 |
| Diabetes mellitus | 13 (17.1) | 7 (13.0) | 6 (27.3) | .180 |
| Malignancy | 10 (13.2) | 9 (16.7) | 1 (4.5) | .265 |
| History of fall | 19 (25.0) | 15 (27.8) | 4 (18.2) | .560 |
| Treatment | ||||
| Beta‐blocker | 26 (34.2) | 18 (33.3) | 8 (36.4) | .796 |
| Angiotensin‐renin system inhibitors | 19 (25.0) | 13 (24.1) | 6 (27.3) | .777 |
Note: Data are median (interquartile range) or number (percentage). P < .05 indicates significant difference between survivors and nonsurvivors.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease.
3.2. Clinical Presentation and Laboratory Findings
The most common symptoms were asthenia (58 cases (76.3%)) and fever (57 cases (75.0%)) (Table 2). The frequency of these symptoms did not differ between survivors and nonsurvivors. Confusion or delirium occurred in 54 cases (71.1%). Confusion was the only symptom in two patients (2.6%). Dyspnea and desaturation were present at admission for 38 patients (50.0%) and for 45 patients (59.2%), respectively. Both were strongly associated to mortality. Indeed, they respectively occurred in 18 cases (81.8%) and 21 cases (95.5%) in nonsurvivors versus 20 cases (37.0%) and 24 cases (44.4%) in survivors (P = .001 and P < .001, respectively). Expectorations, cough, myalgia, and anorexia were also described. Initial fall was reported at the onset of disease or as the motive of admission for 19 patients (25.0%). Digestive symptoms (nausea and vomiting, diarrhea, and abdominal pain) were recorded in 17 cases (22.4%), and were associated with a favorable outcome (29.6% in the survivor group vs 4.5% in the nonsurvivor‐group; P = .029). Agueusia and anosmia were rarely reported, respectively in 4 (5.3%) and 1 (1.3%) patients, as was headache (2 cases (2.6%)), with no significant difference regarding outcome. For the 52 subjects living at home or in a long‐term care facility, the median time from first symptoms to hospital admission was 5 days (IQR, 2–8 days). This delay did not differ significantly between survivors (5 (IQR, 2–8) days) and nonsurvivors (6 (IQR, 2–8) days) (P = .984).
Table 2.
Symptoms, Laboratory Findings, and Imaging
| Onset of symptoms a | Total (N = 52) | Survivors (N = 35) | Nonsurvivors (N = 17) | P value |
| Onset of symptoms to admission, d | 5 (2–8) | 5 (2–8) | 6 (2–8) | .984 |
| Initial symptoms | Total (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Fever | 57 (75.0) | 41 (75.9) | 16 (72.7) | .777 |
| Cough | 42 (55.3) | 29 (53.7) | 13 (59.1) | .800 |
| Expectorations | 23 (30.3) | 15 (27.8) | 8 (36.4) | .583 |
| Dyspnea | 38 (50.0) | 20 (37.0) | 18 (81.8) | .001 |
| Desaturation | 45 (59.2) | 24 (44.4) | 21 (95.5) | <.001 |
| Minimum saturation, % 0 | 90 (87–92) | 92 (89–94) | 89 (87–91) | .023 |
| Digestive symptoms | 17 (22.4) | 16 (29.6) | 1 (4.5) | .029 |
| Asthenia | 58 (76.3) | 38 (70.4) | 20 (90.9) | .076 |
| Myalgia | 11 (14.5) | 9 (16.7) | 2 (9.1) | .494 |
| Ageusia | 4 (5.3) | 4 (7.4) | 0 (0) | .317 |
| Anosmia | 1 (1.3) | 1 (1.9) | 0 (0) | 1.000 |
| Anorexia | 44 (57.9) | 29 (53.7) | 15 (68.2) | .310 |
| Headache | 2 (2.6) | 1 (1.9) | 1 (4.5) | .498 |
| Confusion and delirium c | 54 (71.1) | 37 (68.5) | 17 (77.3) | .580 |
| Inaugural fall | 19 (25.0) | 12 (22.2) | 7 (31.8) | .395 |
| Laboratory findings | Total (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Hemoglobin, g/dL | 12.4 (11.1–13.3) | 12.2 (11.1–13.5) | 12.6 (11.1–13.5) | .740 |
| Lymphocytes, G/L | 0.85 (0.57–1.09) | 0.96 (0.70–1.30) | 0.60 (0.42–0.82) | <.001 |
| Lymphopenia, % | 67 (88.2) | 48 (88.9) | 19 (86.4) | .713 |
| White blood cell count, G/L | 5.85 (4.1–7.57) | 5.75 (4.10–6.90) | 6.20 (3.8–8.8) | .610 |
| Platelets, G/L | 210 (164–255) | 213.5 (168.5–257.5) | 187.5 (157.7–266.2) | .744 |
| Thrombocytopenia, % | 11 (14.5) | 6 (11.1) | 5 (22.7) | .280 |
| Initial C‐reactive protein, mg/dL | 49 (14.5–107.5) | 27 (7–59) | 62.4 (73.5–147.0) | <.001 |
| Apex C‐reactive protein, mg/dL | 65 (32.25–134) | 43 (27–72) | 151.5 (91.0–223.0) | <.001 |
| Procalcitonin, ng/mL | 0.1 (0.05–0.2) | 0.06 (0.04–0.10) | 0.28 (0.16–2.35) | <.001 |
| D‐dimer, ng/mL | 1,170 (745–2,300) | 955 (717.50–1,347.50) | 1,760 (757.5–2,872.5) | .191 |
| Fibrinogen, g/L | 5.49 (4.24–6.36) | 5.2 (4.1–6.3) | 5.7 (4.7–7.2) | .318 |
| Brain‐type natriuretic peptide, pg/mL | 202 (62–476) | 137.5 (44.7–371.2) | 325.0 (137.0–638.0) | .020 |
| Elevated troponin, %b | 16/41 (39.0) | 6/27 (22.2) | 10/14 (71.4) | .003 |
| Initial serum creatinine, μmol/L | 82 (68.25–115.8) | 77.0 (64.50–94.50) | 119.0 (77.5–147.5) | .001 |
| Apex serum creatinine, μmol/L | 88.5 (70–81) | 82.0 (68.0–82.0) | 118.5 (87.0–154.7) | .006 |
| Acute kidney injury, % | 7 (9.2) | 5 (9.3) | 2 (9.1) | >.999 |
| Aspartate aminotransferase, U/L | 35 (27–53) | 32 (24.0–45.75) | 49.0 (33–54.50) | .010 |
| Alanine aminotransferase, U/L | 16 (13–25) | 15.0 (13.0–22.2) | 22.0 (13.0–39.0) | .200 |
| Protein, g/L | 67 (62–74) | 67.0 (62–74) | 67.0 (58–75.50) | .741 |
| Albumin, g/L | 30.9 (27.3–33.9) | 32.3 (29.5–34.7) | 26.5 (23.7–32.0) | .020 |
| Positive viral panel (N = 21) | 0 (0.0) | |||
| Lung imaging (N = 58) | Total (N = 58) | Survivors (N = 35) | Nonsurvivors (N = 23) | P value |
| Bilateral pulmonary infiltration | 41 (70.7) | 25 (71.4) | 16 (69.6) | >.999 |
| Bacterial surinfection | 12 (20.7) | 6 (17.1) | 6 (26.1) | .334 |
| Severity of lesion, lung CT (N = 37) | Total (N = 37) | Survivors (N = 25) | Nonsurvivors (N = 12) | P value |
| Absent | 6 (16.2) | 4 (16.0) | 2 (16.7) | >.999 |
| Slight (<10%) | 11 (29.7) | 9 (36.0) | 2 (16.7) | .279 |
| Moderate (10%–25%) | 10 (27.0) | 8 (32.0) | 2 (16.7) | .445 |
| Wide (25%–50%) | 5 (13.5) | 2 (8.0) | 3 (25.0) | .303 |
| Severe (>50%) | 5 (13.5) | 2 (8.0) | 3 (25.0) | .303 |
| Severity of COVID 19 | Total (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Mild | 12 (15.8) | 12 (22.2) | 0 (0.0) | .015 |
| Moderate | 25 (32.9) | 24 (44.5) | 1 (4.5) | .001 |
| Severe | 39 (51.3) | 18 (33.3) | 21 (95.5) | <.001 |
Note: Data are median (interquartile range) or number (percentage). P < .05 indicates significant differences between survivors and nonsurvivors. Abbreviation: CT, computed tomography.
For 52 subjects who were not admitted at the beginning of symptoms.
Two patients (4.5%) presented with isolated confusion and delirium
Troponin was available for 41 subjects.
Laboratory findings (Table 2) showed lymphopenia in 67 cases (88.2%) and thrombocytopenia in 11 cases (14.5%). C‐reactive protein, procalcitonine, D‐dimers, fibrinogen, and BNP were above their respective normal limit in the whole cohort. Acute kidney injury occurred in 7 cases (9.2%). Nonsurvivors showed lower absolute lymphocyte count at nadir compared with the survivor group (0.60 (IQR, 0.42–0.82) G/L vs 0.96 (IQR, 0.70–1.30) G/L; P < .001). Nonsurvivors also exhibited significantly higher C‐reactive protein (CRP) at admission and follow‐up (respectively 62.4 (IQR, 73.5–147.0) mg/L and 151.5 (IQR, 91.0–223.0) mg/L compared with 27 (IQR, 7–59) mg/L and 43 (IQR, 27–72) mg/L; P < .001). Serum creatinine at admission and follow‐up was significantly higher in nonsurvivors (respectively P = .001 and P = .006). Troponin was above average for 16 of the 41 patients (39.2%) and significantly associated to outcome (P = .003). Median BNP increase was significantly higher in nonsurvivors compared with the survivors (325.0 (IQR, 137.0–638.0) pg/mL vs 137.5 (IQR, 44.7–371.2) pg/mL; P = .020). Albumin serum level was lower in survivors than in nonsurvivors (26.5 (IQR, 23.7–32.0) g/L vs 32.4 (29.5–34.7) g/L; P = .020). There was no significant difference in white blood cell count, hemoglobin, platelet counts, fibrinogen, D‐dimer, and occurrence of acute renal injury regarding outcome between survivors and nonsurvivors. No viral coinfection was found for the 21 patients tested.
Lung imaging was available for 58 patients. Bilateral pulmonary infiltration was reported for 41 patients (70.7%), with no difference between survivors and nonsurvivors (25 patients (71.4%) vs 16 patients (69.6%); P > .999). Severity of lesions on computed tomography scanner tended to be higher in nonsurvivors compared with survivors but did not reach statistical significance (P > .05). Twelve patients (20.7%) presented radiological signs of bacterial surinfection without difference between the two groups.
Severity of COVID‐19 was evaluated as mild for 12 patients (15.8%), moderate for 25 (32.9%), and severe for 39 (51.3%). Nonsurvivors presented with significantly higher frequency of severe form (21 cases (95.5%) vs 18 cases (33.3%) in survivors; P > .001).
3.3. Treatment and Outcome
Treatment related to COVID‐19 was administrated to 60 patients (78.9%) (Table 3), consisting of oxygen therapy by high‐flow nasal canula in 46 (60.5%), antibiotherapy in 50 (65.8%), and specific treatment in 12 (15.8%), associating anti‐inflammatory agents and antivirals. Regarding anti‐inflammatory treatment, glucocorticoids were administered to six patients (7.9%). Five patients (6.6%) received hydroxychloroquine, and one patient (1.3%) received lopinavir/ritonavir. Cardiac decompensation required a specific treatment in 11 patients (14.5%). Nonsurvivors received significantly more frequent oxygen therapy with higher flow (P < .001) and more frequent antibiotherapy with use of beta‐lactamines versus nonsurvivors. ARDS occurred in 22 patients (28.9%). Cardiac decompensation occurred in 11 patients (14.5%), including six nonsurvivors (27.3%) versus five survivors (9.3%) (P = .069). Other complications included hypotensive shock (2 cases (2.6%)) and hyperosmolar coma (2 cases (2.6%)). Case fatality rate at day 21 was of 28.9%. Mortality was higher in men (15/34 (44.1%)) versus women (7/42 (16.7%); P = .0115). Median time from first symptom to death was 13 (IQR, 8–17) days, and median time from admission to death was 6 (IQR, 3–13.5) days. Death occurred secondary to ARDS in 21 cases (95.5%). Associate established causes of death included hyperosmolar coma in two cases (9.0%), hypotensive shock in two cases (9.0%), and cardiac decompensation and metastatic cancer in one case (respectively 4.5%).
Table 3.
Complications, Treatment, and Outcome
| Complications | All patients (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Acute respiratory distress syndrome | 22 (28.9) | 1 (1.9) | 21 (95.5) | <.001 |
| Hypotensive shock | 2 (2.6) | 0 (0.0) | 2 (9.1) | 1.000 |
| Cardiac decompensation | 11 (14.5) | 5 (9.3) | 6 (27.3) | .069 |
| Hyperosmolar coma | 2 (2.6) | 0 (0.0) | 2 (9.1) | 1.000 |
| Treatment | All patients (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Oxygen therapy | 46 (60.5) | 25 (46.3) | 21 (95.5) | <.001 |
| Maximal flow, L/min | 5 (2.75–15) | 3 (1.7–5.0) | 13.5 (5.0–15.0) | <.001 |
| Antibiotherapy | 50 (65.8) | 29 (53.7) | 21 (95.5) | <.001 |
| Betalactamine | 39 (51.3) | 23 (42.6) | 16 (72.7) | .023 |
| Macrolides | 31 (40.8) | 19 (35.2) | 12 (54.5) | .132 |
| Second‐line antibiotherapy | 11 (14.5) | 4 (7.4) | 7 (31.8) | .011 |
| Glucocorticoid therapy | 6 (7.9) | 3 (5.5) | 3 (13.6) | .351 |
| Hydroxychloroquine | 5 (6.6) | 4 (7.4) | 1 (4.5) | 1.000 |
| Lopinavir‐ritonavir | 1 (1.3) | 0 (0.0) | 1 (4.5) | 1.000 |
| Cardiotropic treatment | 11 (14.5) | 5 (9.3) | 6 (27.3) | .069 |
| Curative anticoagulation | 8 (10.5) | 5 (9.3) | 3 (13.6) | .684 |
| Outcome | All patients (N = 76) | Survivors (N = 54) | Nonsurvivors (N = 22) | P value |
| Total mortality at 21 days | 22 (28.9) | |||
| Delay first symptom‐death, d | 13 (8–17) | |||
| Delay admission‐death, d | 6 (3–13.5) | |||
| Length of stay, d | 11 (7–17) | 12 (9–19.5) | 6.5 (3–11.75) | <.001 |
| Discharge before end of follow‐up | 27 (50) |
Note: Data are median (interquartile range) or number (percentage). P < .05 indicates significant differences between survivors and nonsurvivors.
In survivors, median length of stay was 12 (IQR, 9–19.5 days). At the end of follow‐up, 50.0% of patients had been discharged home or in their original long‐term facility. ARDS subsequently occurred in one survivor (1.9%).
3.4. Prognostic Factors
Multivariate Cox proportional hazards regression analysis was performed to identify prognostic factors of death. Seven variables were analyzed, based on the difference observed between survivors and nonsurvivors in univariate analysis (i.e., sex, cardioneurovascular disease history, initial dyspnea, initial desaturation, lymphocytes, initial CRP, initial serum creatinine, and lymphocyte count). Albumin level was excluded from the analysis due to missing values. Multivariate analysis showed that initial CRP level and lymphocyte count on admission were significant independent predictors of mortality at day 21 (Figure 1). Lymphocyte count at nadir was the strongest predictor of death (HR = 0.186; 95% confidence interval (CI) = 0.037–0.932; P = .03). Significance was also found for CRP level at admission (HR = 1.012; 95% CI = 1.005–1.019). A subgroup analysis limited to patients with community‐acquired SARS‐CoV‐2 infection identified the two same predictive factors of death (CRP (HR = 1.011; 95% CI = 1.004–1.019; P = .004) and admission lymphocyte count (HR = 0.083; 95% CI = 0.011–0.605; P = .014; Supplementary Figure S1).
Figure 1.
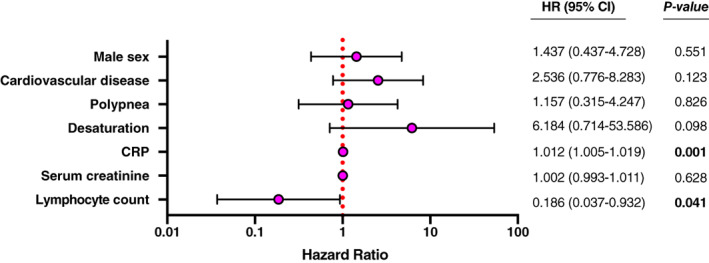
Cox proportional hazard model analysis for death predictive factors at 21 days. CRP, C‐reactive protein; HR, hazard ratio.
4. DISCUSSION
In this study, we report a case series of 76 older adults with confirmed SARS‐CoV‐2 infection and a global frailty with high median age, undernutrition, and numerous comorbidities. COVID‐19 was characterized by specific clinical presentation associating high rates of confusion and fall, a severe infection pattern, with more than half patients presenting with severe pneumonia, and a day 21 mortality rate of 28.9%. Factors associated with death were male sex, history of cardioneurovascular disease, dyspnea and low blood saturation at admission, lymphopenia, elevation of inflammation markers, D‐dimers, BNP, and troponin, and low albumin. Independent predictive factors of death identified were lymphocyte count at nadir and CRP at admission.
Significant clinical features of COVID‐19 associated high frequency of confusion and delirium and frequent occurrence of fall. The literature shows variable rates of confusion associated to COVID‐19 in general population (from 9% 13 to 29.7% 14 ). It has been reported as the only sign of infection by SARS‐CoV‐2 in four cases reports in older adults. 15 , 16 , 17 , 18 Causes of confusion during SARS‐CoV‐2 infection could be due to either direct virus invasion of the central nervous system (CNS) with induction of CNS inflammatory mediators 19 or CNS complications of other organ system failure, effect of sedative strategies, and preexisting cognitive decline. The high rate of confusion in our study, associated with the high prevalence of preexisting cognitive impairment, without any other neurological symptoms nor a proof of direct SARS‐CoV‐2 CNS invasion, suggests that the confusion was rather an unspecific symptom. In regard to falls, in respective series of 17 and 19 patients older than 80 years, Godaert et al 7 and Olde Rikkert et al 20 found similar rates of fall as the one we report (respectively, 23.5% and 32%). Falls were also reported as the first symptom in Olde Rikkert et al and Neerland et al. 7 Both confusion and delirium and falls most likely reflect the frailty state of the patients of our cohort, with vulnerability to poor resolution of homeostasis following an infectious stress. 21 Nevertheless, initial cognitive status and confusion were not associated to outcome in our series. Older subjects are prone to atypical presentation of illnesses, including infectious diseases, and these forms are associated with worse clinical outcome. 22 Awareness on atypical presentation of COVID‐19 in older adults, including isolated confusion, fall, absence of fever, or prominent digestive presentation, will allow for appropriate care without delay and avoid viral spread in medical institution. Likewise, any significant change in clinical status from baseline in older adults with no evident explanation should be evaluated for COVID‐19 infection during the current pandemic.
Subjects of our series had typical signs of viral infection and pneumoniae: fever, dyspnea, cough, and expectoration. In several series, 5 , 6 , 23 older adults could lack signs of viral infection but frequency of those signs in our series did not appear different than observed in general adult population in large analysis. 24 There was no significant difference in the upper respiratory tract symptoms (cough and expectoration) between survivors and nonsurvivors, whereas dyspnea, desaturation, and minimal saturation number were significantly more severe on admission concerning the nonsurvivor patients. 25 The prevalence of digestive symptoms (nausea and vomiting, diarrhea, and abdominal pain) was higher than the one reported in general adult population (15%) in a large meta‐analysis including 7,000 cases. 26 Surprisingly, in our cohort, it was associated with a positive outcome, whereas, in younger adult series, it was associated with more severe symptoms and higher prevalence of complications. The reasons that could explain those differences are not clearly stated yet. 26 , 27
Our patients displayed typical laboratory results reported in COVID‐19. A large majority presented with lymphopenia and, to a lesser extent, thrombopenia, which have been both largely described. 9 Although COVID‐19 has initially been reported as a respiratory syndrome, it is now clear that it affects multiple organs. 28 We observed metabolic complications (kidney injury and diabetes mellitus decompensation) as well as frequent cardiac decompensation. Cardiac failure occurred in 13.2% of the cases, and high BNP level and elevated troponin were associated with mortality. Worsening of previous cardiac condition and coronary heart disease have been reported to be associated to poor outcomes in influenza and other respiratory viral infections. 29 Hypoalbuminenia was associated with poor outcome in our cohort that showed already a high prevalence of malnutrition at baseline. Combined hypoalbuminemia and low BMI has been shown to be a powerful prognostic indicator of high mortality risk in older individuals with limited performance status. 3 , 30 In addition, hypoalbuminemia predicts outcome of COVID‐19 independantly of age and comorbidity. 31
Oxygen therapy was required more often in our series than in large series of younger adult subjects, all form of diseases confounded, probably to be attributed to the severity of disease in regard to age and comorbidities. Antibiotherapy use was similar to the one reported for general adult population 13 and associated to an unfavorable outcome. Anti‐inflammatory drugs, hydroxychloroquine, and antiviral treatment were not associated with outcome but seldom used in our cohort. Variability of treatment in context of frequent contraindications, decision of therapeutic limitations, and changing evidence regarding treatment of COVID‐19 does not allow us to draw any recommendation.
In older patients, mortality rate was variable through the series. Wang et al observed a mortality of 19.2% 5 (339 patients; mean age = 69 years) but the population reported presented with less associated comorbidities than our series. On 5,700 subjects treated in New York for COVID‐19, Richardson et al 9 found a mortality of 60.6% in 155 subjects aged 80 to 89 years and of 63.2% in 44 subjects older than 90 years. Our mortality rate was significantly inferior to the one reported in Richardson's work and is closest to the one observed in general older adult population. Extended delay to hospitalization in Richardson cohort, as the delay of death after hospitalization appears short (median = 3.0 days), 32 could account for this difference.
Our overall cohort presented with numerous factors of negative outcome: comorbidities with preexisting concurrent cardioneurovascular diseases, lymphopenia and thrombocytopenia, high D‐dimers, high inflammation markers, 4 and elevated troponin. Delay between first symptom and death (median = 13 days) was shorter than reported in large series for younger patients, 4 which can be most likely attributed to the frailty of our population and the severity of the disease. Mortality once ARDS occurred was 95.5%, higher than the mortality reported in the adult critically ill population (from 50% to 61.5% in large cohorts 25 , 33 ), explained by the fact that the included patients were not eligible for intensive care.
Median length of hospital stay for the survivors was significantly higher than usually reported, consistent with aging, comorbidities, and lower lymphocyte count observed in our patients. 34 , 35 Interestingly, only two laboratory data were independent predictors of death in the current cohort (i.e., CRP on admission and absolute lymphocyte count). These variables were previously identified as prognosis variables in large cohort of adult patients 4 and in series focusing on older patients. 5 , 6 Why lymphopenia is associated to and predicts mortality is still unclear. We hypothesize that it may result from direct lymphocyte infection by the virus, inflammation leading to apoptosis, or inhibition of lymphocytes by metabolic disorders. 36 Moreover, lymphopenia is known to constitute biological sign of prognostic significance in geriatrics associated with mortality. 37 , 38 CRP is an acute phase reactant and a marker of interleukin‐6–mediated inflammatory reactions. 39 It was reported previously to predict severity in SARS of other origin. 40 COVID‐19 is characterized by a male predominance, which is also associated to higher mortality and severity. 9 , 41 However, as females are overrepresented in the older population, male proportion was lower in our series. It was associated to an unfavorable outcome but did not predict mortality. Diabetes mellitus, hypertension, and cardiovascular or respiratory diseases have also been reported to affect the prognostic of COVID‐19. 4 Cardiovascular diseases were more frequent in the nonsurvivor group but were not identified as a predictor of outcome in multivariate analysis. Our whole population presented with frequent and multiple comorbidities, which may not have enabled us to measure their potential weight on prognosis.
Literature about COVID‐19 mortality in older adults living and cared for in nursing home or long‐term care facility is still scarce. 42 , 43 , 44 , 45 , 46 Mortality ranged from 29% to 47.5%, with variable hospitalization rate 43 , 44 , 45 in available studies, higher that the mortality we observed. 42 , 46 We can hypothethize on a benefit of active treatment with specific care of complications and close monitoring in hospital for oldest patients.
Our study has several limitations, mainly due to its monocentric characteristic and with a midterm follow‐up, thus limiting external validity and long‐term study of COVID‐19. Furthermore, we have enrolled all patients needing hospitalization, including patients still living at home or living in long‐term facility and patients already admitted. This sample heterogeneity could lead to different clinical presentations and risk factors for severe COVID‐19. Finally, the size of the cohort might have limited the ability to identify clinically relevant prognostic factors due to a lack of statistical power.
In conclusion, the current prospective cohort study was one of the largest studies conducted in oldest‐old patients with COVID‐19 infection managed in a real‐life setting. COVID‐19 appears as a severe condition in the older adults with a majority of severe forms and a high 3‐week mortality rate. Distinctive features can be identified in older adults, including multiple organ injuries and atypical clinical presentation. The current study emphasizes the need for early identification and intensive surveillance, leading to appropriate care management of SARS‐CoV‐2 infection. Preventive strategies to avoid transmission to this particularly vulnerable patient setting still remain in great need. Finally, further multicenter studies focusing on this specific population are needed as, despite a higher mortality rate than in younger adults, early diagnosis, close monitoring, and appropriate care of the disease and its complications should benefit the oldest‐old patients.
Supporting information
Supplementary Figure S1: Cox proportional hazard model analysis for predictive factors of death at 21 days in community‐acquired COVID‐19.
ACKNOWLEDGMENTS
LRB COVID Group Collaborators: AMADOR BORRERO Blanca, AVENEAU Clément, BASTARD Paul, BEAUVAIS Diane, BOGHEZ Loredana, BORDERIOU Alix, BURLACU Ruxandra, CHAMPION Karine, CHAUVIN Anthony, CONWAY Paul, COSMA Lavignia, DAVY Vincent, DELCEY Véronique, DESJARDIN Clément, DEVATINE Sandra, DUCROZ GERARDIN Christel, DUPE Charlotte, FERON Florine, FRAZIER Aline, FUNCK‐BRETANO, GALLAND Joris, GAUTHIER Diane‐Cecile, GAUTIER Jean‐François, GOBERT Chloé, GROS Clotilde, HUSCENOT Tessa, JAULERRY Sarah, JOUABLI Moenes, JULLA Jean‐Baptiste, KADIRI Soumaya, KEVORKIAN Jean‐Philippe, KHAN Enmat, LALOI MAWET Jérôme, MICHELIN Marie, LEROY Pierre, LOPES Amanda, MANGIN Olivier, MEGARBANE Bruno, MOULY Stephane, MUNIER Anne‐Lise, ONGNESSEK Sandrine, REVUE Eric, RHMARI Fatima, RICHETTE Pascal, RIVELINE Jean‐Pierre, ROOS Caroline, RUBENSTEIN Emma, SACCO Isabelle, SAPTEFRAT Natalia, SCHAUPP Pauline, SELLIER Pierre‐Olivier, SENE Damien, SERRE Justine, SMATI Sonia, THOREAU Benjamin, TOURNIER Marine, TRECA Pauline, TUFFIER Mathilde, VODOVAR Dominique, ZANIN Adrien.
Conflict of Interest
The authors have no conflicts.
Author Contributions
Agathe Vrillon: conceptualization, data curation, formal analysis, writing‐original draft, writing‐review and editing. Claire Hourregue: conceptualization, data curation, writing‐review and editing. Julien Azuar: conceptualization, data curation, methodology, writing‐review and editing. Lina Grosset, Ada Boutelier, Sophie Tan, Michael Roger, and Vianney Mourman: data curation, writing‐review and editing. Stéphane Mouly, Damien Sène, and Véronique François‐Fasille: conceptualization, validation, writing‐review and editing. Julien Dumurgier: conceptualization, methodology, supervision, validation, writing‐review and editing. Claire Paquet: conceptualization, data curation, methodology, supervision, writing‐original draft, writing‐review and editing.
Sponsor's Role
This work was not sponsored.
‡LRB COVID Group Collaborators are listed in the Acknowledgments.
REFERENCES
- 1. Suzman R, Riley MW. Introducing the “oldest old”. Milbank Mem Fund Q Health Soc. 1985;63(2):177‐186. [PubMed] [Google Scholar]
- 2. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80:639‐645. 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID‐19 in elderly patients: a comparison with young and middle‐aged patients. J Infect. 2020;80(6):e14‐e18. 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Godaert L, Proye E, Demoustier‐Tampere D, Coulibaly PS, Hequet F, Dramé M. Clinical characteristics of older patients: the experience of a geriatric short‐stay unit dedicated to patients with COVID‐19 in France. J Infect. 2020;81(1):e93‐e94. 10.1016/j.jinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in new York City: a prospective cohort study. medRxiv. 2020;395 1763–1770. 10.1101/2020.04.15.20067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the new York City area. JAMA. 2020;323:2052‐2059. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205‐206. 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 11. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 12.Clinical management of severe acute respiratory infection when COVID‐19 is suspected. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed May 15, 2020.
- 13. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7:611‐627. 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neerland BE, Dobloug A, Nore KG, Mikaelsen EE, Halsen A, Ahmed MV. COVID‐19 in an elderly woman with acute functional decline. Tidsskr Nor Laegeforen. 2020;140(7). 10.4045/tidsskr.20.0307. [DOI] [PubMed] [Google Scholar]
- 16. Alkeridy WA, Almaglouth I, Alrashed R, et al. A unique presentation of delirium in a patient with otherwise asymptomatic COVID‐19. J Am Geriatr Soc. 2020;68 1382–1384. 10.1111/jgs.16536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tay HS, Harwood R. Atypical presentation of COVID‐19 in a frail older person. Age Ageing. 2020;49(4):523‐524. 10.1093/ageing/afaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butt I, Sawlani V, Geberhiwot T. Prolonged confusional state as first manifestation of COVID‐19. Ann Clin Transl Neurol. 2020;7(8):1450‐1452. 10.1002/acn3.51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saleki K, Banazadeh M, Saghazadeh A, Rezaei N. The involvement of the central nervous system in patients with COVID‐19. Rev Neurosci. 2020;31(4):453‐456. 10.1515/revneuro-2020-0026. [DOI] [PubMed] [Google Scholar]
- 20. Olde Rikkert MGM, Vingerhoets RW, van Geldorp N, de Jong E, Maas HAAM. [Atypical clinical picture of COVID‐19 in older patients]. Ned Tijdschr Geneeskd. 2020;164. [PubMed] [Google Scholar]
- 21. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752‐762. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hofman MR, van den Hanenberg F, Sierevelt IN, Tulner CR. Elderly patients with an atypical presentation of illness in the emergency department. Neth J Med. 2017;75(6):241‐246. [PubMed] [Google Scholar]
- 23. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu J, Zhong Z, Ji P, et al. Clinicopathological characteristics of 8697 patients with COVID‐19 in China: a meta‐analysis. Fam Med Commun Health. 2020;8(2):e000406. 10.1136/fmch-2020-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao R, Qiu Y, He J‐S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5:798‐799. 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115:766‐773. 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol. 2020;1449‐1459. 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corrales‐Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496‐505. 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 30. Lai K‐Y, Wu T‐H, Liu C‐S, et al. Body mass index and albumin levels are prognostic factors for long‐term survival in elders with limited performance status. Aging (Albany NY). 2020;12(2):1104‐1113. 10.18632/aging.102642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Cheng A, Kumar R, et al. Hypoalbuminemia predicts the outcome of COVID‐19 independent of age and co‐morbidity. J Med Virol. 2020;92. 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. French Ministery of Health and Solidarity . Guidelines and recommandation regarding the support of hospital care system to nursing home. https://solidarites-sante.gouv.fr/IMG/pdf/strategie-prise-en-charge-personnes-agees-covid-19.pdf. Accessed March 31, 2020.
- 33. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu X, Zhou H, Zhou Y, et al. Risk factors associated with disease severity and length of hospital stay in COVID‐19 patients. J Infect. 2020;81(1):95–97. 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hong Y, Wu X, Qu J, Gao Y, Chen H, Zhang Z. Clinical characteristics of coronavirus disease 2019 and development of a prediction model for prolonged hospital length of stay. Ann Transl Med. 2020;8(7):443. 10.21037/atm.2020.03.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moulias R, Proust J, Rosenzweig P, Nafziger J, Marescot MR, Lesourd B. [Lymphopenia and bacterial infections: a biological sign of major prognostic significance in geriatrics]. Presse Med. 1986;15(4):166. [PubMed] [Google Scholar]
- 38. Proust J, Rosenzweig P, Debouzy C, Moulias R. Lymphopenia induced by acute bacterial infections in the elderly: a sign of age‐related immune dysfunction of major prognostic significance. Gerontology. 1985;31(3):178‐185. 10.1159/000212700. [DOI] [PubMed] [Google Scholar]
- 39. Pepys MB, Hirschfield GM. C‐reactive protein: a critical update. J Clin Invest. 2003;111(12):1805‐1812. 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang J‐T, Sheng W‐H, Fang C‐T, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10(5):818‐824. 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81(2):16–25. 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan LF, Seetharaman SK. COVID‐19 outbreak in nursing homes in Singapore. J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brouns SH, Brüggemann R, Linkens AEMJH, et al. Mortality and the use of antithrombotic therapies among nursing home residents with COVID‐19. J Am Geriatr Soc. 2020;68(8):1647–1652. 10.1111/jgs.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS‐CoV‐2 infection and COVID‐19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020. 10.1093/cid/ciaa763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trabucchi M, De Leo D. Nursing homes or besieged castles: COVID‐19 in northern Italy. Lancet Psychiatry. 2020;7(5):387‐388. 10.1016/S2215-0366(20)30149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Cox proportional hazard model analysis for predictive factors of death at 21 days in community‐acquired COVID‐19.


