Abstract
To date, little is known about the duration and effectiveness of immunity as well as possible adverse late effects after an infection with SARS-CoV-2. Thus it is unclear, when and if liver transplantation can be safely offered to patients who suffered from COVID-19. Here, we report on a successful liver transplantation shortly after convalescence from COVID-19 with subsequent partial seroreversion as well as recurrence and prolonged shedding of viral RNA.
KEYWORDS: clinical research/practice, immunosuppressant, immunosuppression/immune modulation, liver allograft function/dysfunction, liver transplantation/hepatology, organ transplantation in general, T cell biology
1. CASE REPORT
A 56-year-old male patient was listed for liver transplantation with a Model For End-Stage Liver Disease (MELD) Score of 19 points due to cryptogenic cirrhosis and a history of hepatitis B. Transplantation was considered urgent, as the patient suffered from tense ascites with recurring spontaneous bacterial peritonitis, esophageal varices, acute-on-chronic renal failure, and newly occurring dyskinesia that required repeated periods of hospitalization. The patient was readmitted to our hospital due to exacerbation on March 18, 1 week after COVID-19 was declared a pandemic by the WHO. At that time however, the pandemic was still in its very early phase in Germany, with only 9360 confirmed cases in the entire country with a population of about 83 million. The hygienic protective measures and test capacities were thus still in the process of development at our institution. These circumstances promoted the risk of nosocomial infection with SARS-CoV-2, which unfortunately was the case in our patient. A nasopharyngeal swab confirmed infection by PCR on March 25 ( Figure 1B). Waiting list status was changed to “nontransplantable,” and he was transferred to a COVID-19 isolation ward where he developed increasing malaise and dry cough.
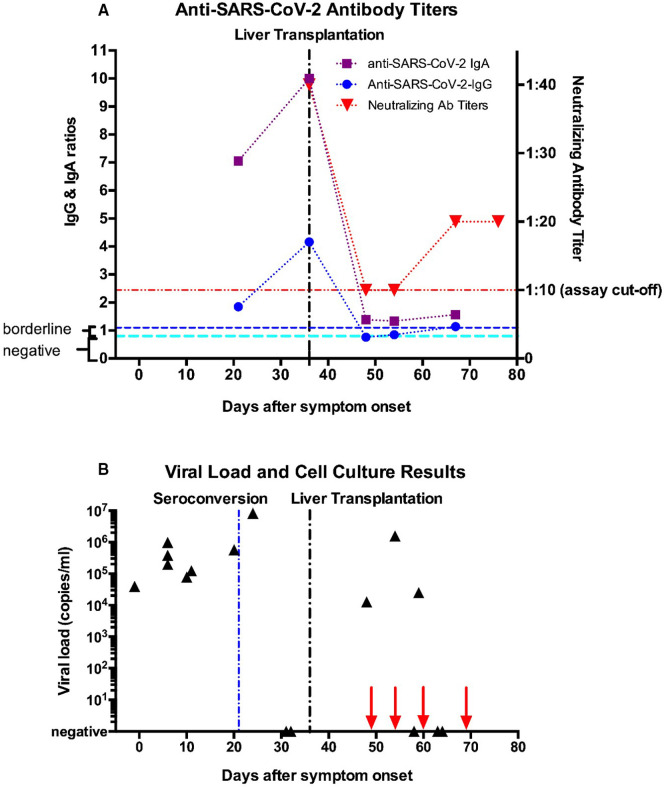
FIGURE 1.
Timeline of test results for (A) antibody levels and (B) viral load and live virus cell culture. (A) Semiquantitative results using the Euroimmune Anti-SARS-CoV-2-ELISA for IgG and IgA targeting the spike domain S1 are indicated (result interpretation ranges as indicated by the manufacturer: negative = 0–0.8 [cyan line], borderline = 0.8–1.1, positive = above 1.1 [blue line]; the coefficient of variation is <10% for positive samples, as stated by the manufacturer). Swab technique and type used were the same for all tests. Neutralizing antibody titers from a microneutralization assay are shown on the right y-axis. The lower assay cut-off of 1:10 is indicated by the red line. (B) Viral load results (black triangles) are shown on a logarithmic scale with copy number estimates expressed as SARS-CoV-2 RNA copies per ml respiratory sample using the CDC N1 reaction as previously described.11 Copy numbers were calculated based on a standard curve using serial dilutions of a plasmid containing the N-gene with known copy numbers (IDT) on a Roche Light Cycler II 480. High variability of test results around day 60 most likely arise from suboptimal swab technique. Live virus cell culture results were all negative, dates are indicated by red arrows on the x-axis [Color figure can be viewed at wileyonlinelibrary.com]
Overall the patient had a mild course of COVID-19. Vital signs remained stable and lab work was unremarkable except for mild lymphopenia. In the absence of dyspnea, a thoracic CT scan was not performed. The patient received no COVID-19-specific or antiviral therapy. SARS-CoV-2 RNA remained detectable in routine swabs after seroconversion (anti-SARS-CoV-2 IgG and IgA antibodies) had occurred on the 21st day after onset of symptoms (Figure 1A). Persistence of viral RNA shedding despite seroconversion was recently described.1 It was not until the 31st and 32nd day of illness that nasopharyngeal swabs showed negative PCR results. The patient was free of COVID-19 symptoms, thus isolation was ended and he was transferred to a normal ward without isolation.
The questions of when the patient would be able to be transplanted and what additional risks the recent diagnosis of COVID-19 would pose were discussed at the interdisciplinary transplantation conference under consideration of the sparse existing data on this topic at the time.2 , 3 Due to the complete recovery of the patient with proven seroconversion and two negative PCR results for viral RNA, the burden of liver disease in this seriously ill patient was deemed to outweigh potential risks of the resolved SARS-CoV-2 infection.
On 1st of May, 36 days after onset of COVID-19 symptoms and 15 days after documented seroconversion, the patient received an organ offer of a 26-year-old brain dead donor, tested negative for SARS-CoV-2. Preoperatively, our patient remained asymptomatic and afebrile. Laboratory testing showed no inflammatory changes, and in particular white blood cell count was normalized. To detect residual signs of COVID-19, a thoracic CT was performed prior to surgery, which showed sporadic and slight thickening of bronchial walls, but no radiographic signs indicative of COVID-19 pneumonia. Since the preoperative examination revealed no contraindications, we proceeded with the transplantation. Both the transplantation and the postoperative course were free of complications. With a history of hepatitis B but absent anti-HBs antibodies (anti-HBcAb: positive, HBsAg: negative, HBV-DNA PCR: negative) the patient received anti-hepatitis B immunoglobulin intraoperatively and Entecavir postoperatively. Standard immunosuppression was initiated with low dose tacrolimus with a target level of 4–7 ng/ml and steroids. The initial Tacrolimus levels were deliberately kept low (3.9 ng/ml measured) to avoid over-immune suppression as far as possible. Steroids were rapidly tapered. During the entire postoperative course the patient did not suffer from any infections.
As this patient appears to be the first one—to our knowledge—to receive a liver transplant shortly after resolved COVID-19 infection, we sought to gain more insight into the effect the procedure and immunosuppression might have on viral status and antibody titers. To our surprise, SARS-CoV-2 RNA was recurrently detected in upper respiratory swabs during routine testing at viral loads comparable to pretransplantation levels on days 27, 33, and 38 after seroconversion, while the patient remained asymptomatic and afebrile the whole time. To further explore this unusual case, we also repeated antibody testing, which showed partial seroreversion with now seronegativity for anti-SARS-CoV-2 IgG antibodies with persistingly low levels of IgA. Subsequent testing returned only borderline positive results for IgG (blue line, Figure 1A) and very low positive IgA levels (purple line, Figure 1A). Neutralizing antibody levels, measured using a microneutralization assay (red line, Figure 1A), also declined markedly after liver transplantation from mid-low range titer of 1:40 to very low 1:10 titers. However, viral infectivity appeared to be absent as four subsequent attempts of live viral isolation in cell culture all returned negative (red arrows, Figure 1B). We ruled out reinfection with a different viral strain by near-full length SARS-CoV-2 genome sequencing of samples before and after liver transplantation, which proved to be identical (data not shown). After two further negative swab results, the patient was discharged on postoperative day 33 with excellent liver function and no COVID-19 symptoms. He continued to show no signs of COVID-19 by the time this article was written.
2. DISCUSSION
This case addresses the questions of if and when a patient can be safely transplanted after recovery from COVID-19. In approaching this question, we considered two aspects: first, is the patient at increased risk for recurrence of the disease, potentially in a severe form, during the perioperative phase and second, is the previously acquired immunity against COVID-19 compromised by the treatment?
Although the general outcome of liver transplantation was favorable, the impact of surgery and immunosuppression on immunity against SARS-CoV-2 infection remains unclear. Our decision to perform liver transplant was based on the conditions that the need for transplantation was high, all COVID-19 symptoms had subsided, viral clearance and seroconversion were detected and structural pulmonary residuals were absent (exclusion in CT scan). The recommendations of the WHO for clinical management of COVID-19, which demands two negative PCRs prior to discharge from the hospital, were met in our patient before transplantation. Nonetheless, recurrence and prolonged detection of viral RNA as well as rapid loss of IgG seropositivity and decline of neutralizing antibody titers are potential sequelae of transplantation that might prove disadvantageous. We consider blood loss and subsequent hemodilution by i.v. fluids and blood products as well as possibly the effects of immunosuppression to be the main contributors to the variability in antibody levels we observed. The true implications of these findings for the patient are unclear, however, as the exact mechanisms by which immunity against SARS-CoV-2 is mediated are not yet fully understood.4 In particular, foundational knowledge about how the balance and phenotypes of responding cells vary as a function of disease course and severity is still missing for COVID-19. Thus, there is great uncertainty about the temporal duration of protective immunity and about the role each component of the adaptive immune system plays herein. For instance, although development of profound circulating antibody levels against the virus has been shown in a large proportion of COVID-19 patients, recent reports suggest an early decline of IgG and neutralizing antibody levels after 2–3 months combined with prolonged viral RNA shedding, especially in asymptomatic patients.5 These findings closely resemble what we observed in our patient, but stand in contrast with the comparably long-lasting seropositivity observed after the SARS pandemic of 2003, caused by SARS-CoV-1, a virus with a high degree of similarity to SARS-CoV-2.6 These data suggest only short-lasting protective immunity mediated by antibodies, which is supported by mathematical models on the course of the pandemic.7
On the other hand, a profound virus-specific T cell response elicited by SARS-CoV-2 was recently discovered in a large cohort. SARS-CoV-2 reactive CD4+ and CD8+ T cells were detected in 100% and 70% of convalescent patients, respectively.4 Whether this reaction leads to a long-lived memory T cell response that confers long-term immunity against the disease is unclear, although this has been shown to be the case in SARS-CoV-1.8 Unfortunately, we did not assess T cell reactivity in our patient. Thus it remains uncertain, if this aspect of immunity is present in our patient and if so, whether it is jeopardized by the immunosuppression he receives. From the sparse literature on the topic it is known that memory T cell function against other viruses, such as CMV, seems not to be affected by aggressive induction regimens of immunosuppression.9
The possibility of reinfection after recent COVID-19 remains controversial despite over 5 000 000 patients who recovered from the disease worldwide. This observation has been sustained by the lack of evidence of reinfection in rhesus macaques that were challenged again with the virus after convalescence.10 Although our patient tested positive on nasopharyngeal swabs and sputum again following transplantation, re-infection appears unlikely as the patient suffered no symptoms, viral sequencing showed identical RNA sequences, and the virus failed to replicate in cell culture. If the rapid decline in protective antibody levels we observed in our patient truly reflects detrimental effects on immunity is unknown, as memory T cell response might still be unaffected and potentially prove to be the more important pillar of immunity against COVID-19. In summary, despite worrisome aspects in the postoperative course, we are convinced that the recent COVID-19 did not put the patient at an increased risk during the liver transplantation he urgently needed.
ACKNOWLEDGMENT
Open access funding enabled and organized by ProjektDEAL.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding information ProjektDEAL
REFERENCES
- 1.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489–1501 e1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Fontanet A, Zhang PH, et al. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis. 2006;193(6):792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng H, Yang LT, Wang LY, et al. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351(2):466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranavova L, Hruba P, Girmanova E, et al. The effect of induction therapy on established CMV specific T cell immunity in living donor kidney transplantation. Physiol Res. 2018;67(2):251–260. doi: 10.33549/physiolres.933736. [DOI] [PubMed] [Google Scholar]
- 10.Deng W, Bao L, Liu J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369(6505):818–823. doi: 10.1126/science.abc5343. eabc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muenchhoff M, Mairhofer H, Nitschko H, et al. Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Euro Surveill. 2020;25(24):2001057. doi: 10.2807/1560-7917.ES.2020.25.24.2001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.