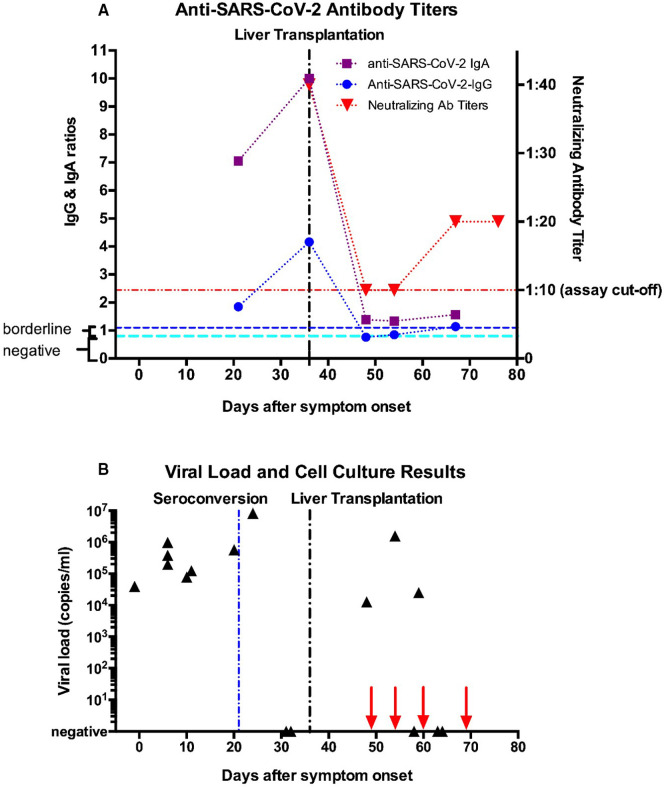
FIGURE 1.
Timeline of test results for (A) antibody levels and (B) viral load and live virus cell culture. (A) Semiquantitative results using the Euroimmune Anti-SARS-CoV-2-ELISA for IgG and IgA targeting the spike domain S1 are indicated (result interpretation ranges as indicated by the manufacturer: negative = 0–0.8 [cyan line], borderline = 0.8–1.1, positive = above 1.1 [blue line]; the coefficient of variation is <10% for positive samples, as stated by the manufacturer). Swab technique and type used were the same for all tests. Neutralizing antibody titers from a microneutralization assay are shown on the right y-axis. The lower assay cut-off of 1:10 is indicated by the red line. (B) Viral load results (black triangles) are shown on a logarithmic scale with copy number estimates expressed as SARS-CoV-2 RNA copies per ml respiratory sample using the CDC N1 reaction as previously described.11 Copy numbers were calculated based on a standard curve using serial dilutions of a plasmid containing the N-gene with known copy numbers (IDT) on a Roche Light Cycler II 480. High variability of test results around day 60 most likely arise from suboptimal swab technique. Live virus cell culture results were all negative, dates are indicated by red arrows on the x-axis [Color figure can be viewed at wileyonlinelibrary.com]