Figure 2.
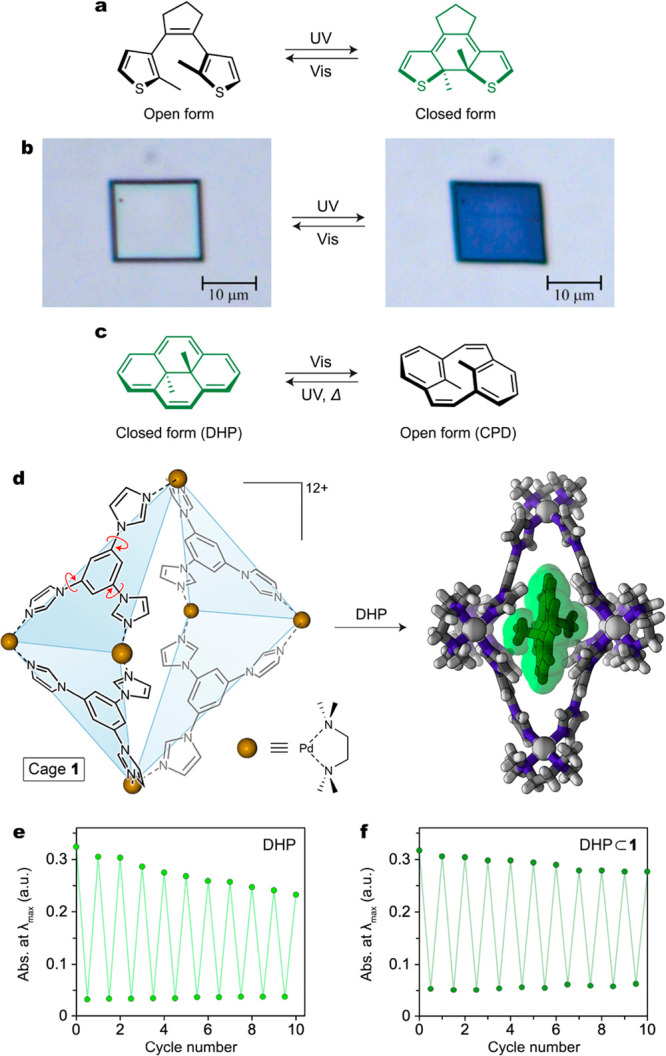
(a) Reversible photoisomerization of a diarylethene. The colored isomer featuring extensive conjugation of π electrons (here, the closed form) is shown in green. (b) Reversible light-induced deformation of a single crystal of a simple diarylethene (here, 1,2-bis(2-ethyl-5-phenyl-3-thienyl)perfluorocyclopentene). (c) Reversible photoisomerization between dihydropyrene (DHP) and cyclophanediene (CPD). (d) Structural formula of coordination cage 1 used to investigate the behavior of photoswitchable molecules under confinement (left) and crystal structure of an inclusion complex of DHP inside cage 1 (right). (e) Gradual decomposition of DHP in pentane solution over 10 switching cycles. (f) Improved fatigue resistance of DHP⊂1 over 10 cycles under the same irradiation conditions. (b) Adapted with permission from ref (24). Copyright 2007 Springer Nature. (e, f) Adapted from ref (31). Copyright 2020 American Chemical Society.
