Conspectus
The electron’s spin, its intrinsic angular momentum, is a quantum property that plays a critical role in determining the electronic structure of molecules. Despite its importance, it is not used often for controlling chemical processes, photochemistry excluded. The reason is that many organic molecules have a total spin zero, namely, all the electrons are paired. Even for molecules with high spin multiplicity, the spin orientation is usually only weakly coupled to the molecular frame of nuclei and hence to the molecular orientation. Therefore, controlling the spin orientation usually does not provide a handle on controlling the geometry of the molecular species during a reaction. About two decades ago, however, a new phenomenon was discovered that relates the electron’s spin to the handedness of chiral molecules and is now known as the chiral induced spin selectivity (CISS) effect. It was established that the efficiency of electron transport through chiral molecules depends on the electron spin and that it changes with the enantiomeric form of a molecule and the direction of the electron’s linear momentum. This property means that, for chiral molecules, the electron spin is strongly coupled to the molecular frame. Over the past few years, we and others have shown that this feature can be used to provide spin-control over chemical reactions and to perform enantioseparations with magnetic surfaces.
In this Account, we describe the CISS effect and demonstrate spin polarization effects on chemical reactions. Explicitly, we describe a number of processes that can be controlled by the electron’s spin, among them the interaction of chiral molecules with ferromagnetic surfaces, the multielectron oxidation of water, and enantiospecific electrochemistry. Interestingly, it has been shown that the effect also takes place in inorganic chiral oxides like copper oxide, aluminum oxide, and cobalt oxide. The CISS effect results from the coupling between the electron linear momentum and its spin in a chiral system. Understanding the implications of this interaction promises to reveal a previously unappreciated role for chirality in biology, where chiral molecules are ubiquitous, and opens a new avenue into spin-controlled processes in chemistry.
Key References
Rosenberg R.; Abu Haija M.; Ryan P. J.. Chiral-selective Chemistry Induced by Spin-polarized Secondary Electrons from a Magnetic Substrate. Phys. Rev. Lett. 2008, 101, 178301..1This work shows for the first time that a polarized spin can induce an enantiospecific chemical process.
Kumar A.; Capua E.; Kesharwani M. K.; Martin J. M. L.; Sitbon E.; Waldeck D. H.; Naaman R.. Chirality-induced Spin Polarization Places Symmetry Constraints on Biomolecular Interactions. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 2474–2478.Here it is shown for the first time that charge polarization in chiral molecules is accompanied by spin polarization.
Banerjee-Ghosh K.; Ben Dor O.; Tassinari F.; Capua E.; Yochelis S.; Capua A.; Yang S.-H.; Parkin S. S. P.; Sarkar S.; Kronik L.; Baczewski L. T.; Naaman R.; Paltiel Y.. Separation of Enantiomers by Their Enantiospecific Interaction with Achiral Magnetic Substrates. Science 2018, 360, 1331–1334.This is the first work on enantiomer separation and chemistry using the CISS effect.
Ziv A.; Saha A.; Alpern H.; Sukenik N.; Baczewski L. T.; Yochelis S.; Reches M.; Paltiel Y.. AFM-Based Spin Exchange Microscopy Using Chiral Molecules. Adv. Mater. 2019, 31, 1904206..4The first direct measurement of spin exchange interactions with chiral molecules is presented here.
Introduction
Electron spin is essential for understanding chemical bonding, but the use of spin-control in synthetic chemistry is not widespread.5 Although the field of “Spin Chemistry” is growing,6 spin effects are usually associated with radical pair reactions. Even though radical chemistry represents a general class of chemical reactivity and radical pair reactions display significant magnetic field effects,7 spin considerations do not typically provide stereospecificity in bimolecular reactions or in reactions between radicals and surfaces. As two molecular radicals react, it is usually not possible to control the relative orientation of the interacting spins and their orientation with respect to the reaction coordinate, because of the weak coupling of the electron spin with the molecule’s nuclear geometry. Even if an external magnetic field is applied to orient the spins in the laboratory frame, they are largely decoupled from the molecular frame. In addition, it is often assumed that spin control is not important for synthetic chemistry because of the small energy associated with spin flipping. This latter reasoning is less rigorous, however, as electron spin relaxation time scales are long compared to typical electronic and vibrational time scales in molecules and often commensurate or as long as rotational time scales.
Hence, it is often assumed that spin considerations are of little relevance to most bimolecular interactions or for the interaction of molecules with surfaces. It must be appreciated that support for this notion arises from the small spin–orbit coupling typical of organic molecules. This small value means that spin does not contribute significantly to the total angular momentum of molecular collisions, even in cases where angular momentum might be used to control chemical processes.8 While this view holds for achiral molecules, recent experiments (vide infra) show that chiral molecules and chiral catalysts display prominent spin selectivity effects.9
An important and well-known exception to the above claim is the case of photodissociation, as well as other photochemical reactions.5−10 Especially one should note the role of spin in the electron transfer dynamics in Photosystem I11 and the role spin may play in the avian compass.12 In photochemistry and photoinduced reaction dynamics studies, it often becomes possible to prepare the reactant electronic state so that the electron spins on the product fragments are oriented.13,14 It is important to note that what maintains the relative orientation of the two spins is the “spin exchange interaction”, which can be many times kBT at room temperature.
Below we discuss experiments that show that spin constraints can play a major role for reactions involving chiral molecules. Our discussion begins by describing the chiral induced spin selectivity (CISS) effect, which provides the connection between a molecule’s chiral structure and the electron spin. Next, we provide several examples for how the CISS effect leads to spin-controlled reactions and interactions. Because the spin degree of freedom is coupled to the linear momentum of the electron for chiral molecules, electron transfer and electron rearrangement of chiral reactants (and intermediates) are affected by the spin. These features mean that CISS is relevant also in biology.15
The CISS Effect
About two decades ago, it was found that electron transmission through chiral molecules is spin dependent; that is, chiral molecules can act as spin filters.16 Since that time, the effect has been reported for many chiral molecules, chiral polymers, chiral organic–inorganic assemblies, and chiral inorganic materials, by numerous groups.17 Important new insights about the importance of the CISS effect in Chemistry and the enantiospecific interaction of molecules arose recently when the CISS effect was also found to manifest as a transient spin polarization, which accompanies charge polarization in chiral molecules.2,3 While it is broadly appreciated that charge reorganization occurs for a molecule under an applied electric field or when a molecule interacts with another molecule or with a surface, the concomitant spin polarization for chiral molecules has not been appreciated before.
Consider a closed shell molecule (total spin zero). Charge polarization of the molecule generates a dipole moment by creating excess charge density in one region of the molecule and depleting charge density in the opposite region, that is, the positive and negative poles of the dipole. For chiral molecules, the electron displacement current generates a spin dipole. Which spin is associated with which electrical pole depends on the handedness of the molecule (see Figure 1). While the total spin of the molecule remains zero, the spin dependent charge reorganization (SDCR) generates spin polarization with opposite signs at the two electric poles. The SDCR affects the reactivity of chiral molecules and may contribute to enantioselectivity in the interaction between chiral molecules. The SDCR also affects the interaction of chiral molecules with ferromagnetic surfaces,18 and it has been used for separating enantiomers.3
Figure 1.
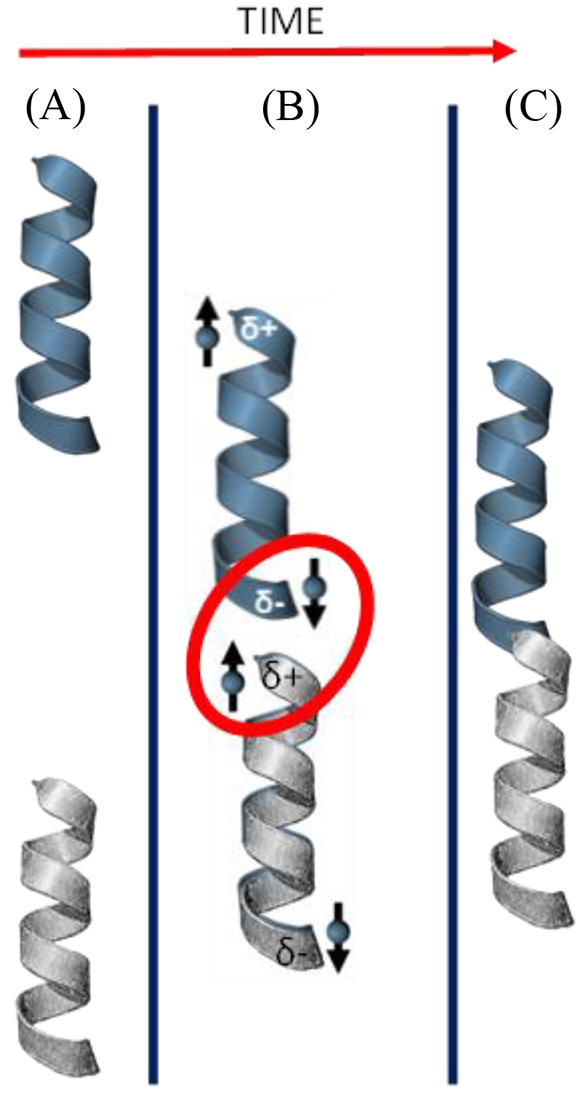
Spin dependent charge reorganization (SDCR) effect. (A) Two chiral reactant molecules are represented by helices. (B) As the chiral molecules approach each other, dispersion forces generate induced dipoles on each molecule. Because of the SDCR effect, the charge polarization is accompanied by a spin polarization. In the case of homochiral reactants, the spins on the opposite electric poles are antiparallel (singlet configuration), while if the molecules are of opposite handedness, the spins on the opposite poles will be parallel (triplet configuration). (C) The two chiral molecules react to give a product. If the spin polarizations on the molecules are antiparallel, then a singlet state is formed, and if the spin polarizations are parallel, a triplet state is formed. Since commonly the singlet state is more energetically favorable, the reaction between homochiral molecules is more favorable as compared to reaction between heterochiral molecules.
The SDCR effect implies that closed shell chiral molecules can have their electron spin distribution polarized relative to the molecular axis, analogous to a “singlet biradical”, but with no need for an external magnetic field. This property opens a new route into spin selective chemistry.
Hall Measurements
A Hall effect device19 is commonly used to measure magnetic fields. In the device, current is flowing between source and drain electrodes and a magnetic field acts on the device. The magnetic field and the current generate a Lorentz force acting on the charges, which creates an electric potential (the Hall voltage) perpendicular to the current and the magnetic field. Thus, the magnitude of the Hall potential reports on the strength of the magnetic field. In our case, we used a Hall device to measure directly the SDCR effect, by placing a self-assembled monolayer film of α-helical peptide oligomers on the surface of a Hall device and measuring the film’s magnetization as a function of the voltage applied across the peptides, the length of the peptide oligomer, and the chirality (l versus d) of the oligomer.20 In these measurements, no external magnetic field was applied; rather the SDCR effect in the peptide monolayer generates a magnetic field at the Hall device surface producing a Hall voltage.
Figure 2 illustrates a Hall device, comprising a GaN/AlGaN structure with a two-dimensional electron gas layer. The Hall bar circuit is buried under a protective 20 nm film so that it can be used as a working electrode in an electrochemical cell. This device is used to study the SDCR in the chiral polymers [poly(4-ethynylbenzoyl-l-alanine decyl ester) (poly-1L), poly(4-ethynylbenzoyl-d-alanine decyl ester) (poly-1D), and poly(4-ethynylbenzoyl-2-methylalanine decyl ester) (poly-2)].21 By applying a constant current between the source and drain electrodes of the Hall bar circuit, one can monitor the magnetization on the working electrode. Although no external magnetic field is applied, a magnetic field is generated by the SDCR effect as a voltage is applied across the chiral polymers, which are adsorbed on the surface of the device.
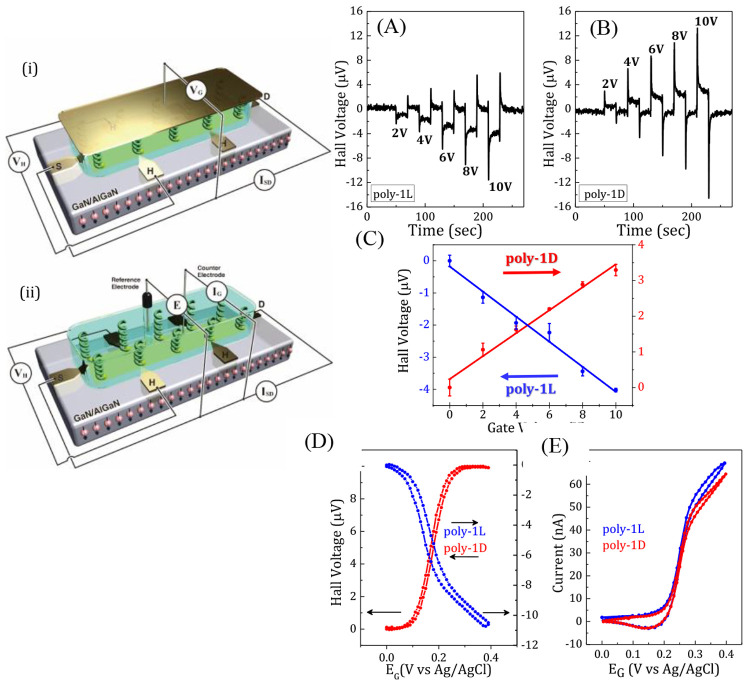
Figure 2.
Schematic presentation of the experimental set of Hall measurement in the polarization (i) and electrochemical mode (ii). Hall potential recorded in polarization mode as a function of time for (A) poly-1L and (B) poly-1D for various gate pulses. (C) The Hall voltage as a function of the gate voltage for monolayers of poly-1L (blue) or poly-1D (red). (D) Hall potential recorded in the electrochemical mode as a function of the voltage when the working electrode (the Hall device) is coated with monolayers of poly-1L (blue) or poly-1D (red). (E) The voltammograms are shown for a working electrode that is coated with monolayers of poly-1L (blue) or poly-1D (red). Note that all electrochemical measurements were performed using ferrocene as a redox probe in water. A Pt wire was used as the counter electrode, the drain electrode of the Hall device was used as the working electrode, and a silver wire was used as a reference electrode. Reprinted with permission from ref (21). Copyright 2020 Wiley-VCH.
Two types of measurements are shown in Figure 2. In each case, the polymers are adsorbed on the device, which is placed within an electrolyte solution and used as a “smart” working electrode. In the first type of experiment, an inert electrolyte is used and a voltage is applied between the “smart” working electrode and a Au counter electrode. Upon application of a voltage, the polymer’s electron cloud polarizes, and this charge polarization is accompanied by spin polarization. The spins that are exchanged between the polymer and the working electrode’s surface generate a magnetization (Figure 2i). The mechanism by which this device operates is described in detail in ref (22). In the second type of experiment, the spin-dependence of the Faradaic current in a redox reaction was measured (Figure 2ii). Here, the Hall device served as the working electrode in a three-electrode electrochemical cell with a Pt counter electrode and a Ag/AgCl reference electrode. When Faradaic current flows to the working electrode through the chiral polymer’s film, the current is spin-polarized, thus generating a magnetization and a Hall voltage. In this scheme, one can simultaneously measure the charge current versus applied potential (cyclic voltammogram) and the magnetization versus applied potential (Hall potential versus voltage) for the redox reaction. It is important to appreciate the difference between the two modes of experiments using the Hall device. In the first type of measurement, the spin injected into the Hall device arises from transient charging (double-layer) current, whereas the second type of experiment has a steady-state current flowing from the Faradaic process.
Figure 2A,B shows the Hall signals as a function of time that were obtained by the first type of measurement for poly-1L and poly-1D on the Hall device. The signals shown are the Hall voltages that were measured for different biases (gate voltages), applied between the counter electrode and the “smart” working electrode. The magnitude of the measured Hall potential is proportional to the voltage applied (see Figure 2C), and the sign of the Hall potential depends on the handedness of the polymers. These results confirm the SDCR process.
In the second type of experiment, we measured the spin selective electron transfer through the polymers for a ferrocene redox couple in a phosphate-buffered saline (PBS) solution. Figure 2D shows the Hall potential obtained when the potential in the electrochemical cell was varied between 0 and 0.4 V. The corresponding cyclic voltammograms are shown in Figure 2E. While the voltammograms for the two enantiomers are very similar, the Hall potentials that are measured have opposite signs. Note the different axes for the l and d polymers. The small hysteresis observed in the Hall potential plots indicates asymmetry between the spin polarization in the oxidation and reduction currents that may result from differences in the interfaces on the two sides of the polymers, one being the solid substrate and the other the electrolyte. These experiments, as well as others, demonstrate spin selective electron transport through chiral films adsorbed on electrodes. This approach for producing spin-polarized electron current opens a new way for performing spin selective electrochemical reactions, vide infra.
Interaction of Chiral Molecules with Ferromagnetic Surfaces
The spin-dependent charge reorganization observed in chiral molecules implies that the interaction between a chiral molecule and a magnetized surface should be enantiospecific. Consider a ferromagnetic metal that is magnetized along its surface normal so that the spin sub-bands of the conduction electrons are split in energy, presenting more filled orbitals of one spin direction and more empty orbitals of the other spin direction. Because of the spin-dependent metal orbital population, the chemisorption or physisorption of a molecule will depend on whether the molecule’s orbitals have a preferred spin direction with respect to the metal. For example, the interaction energy for a molecule forming a chemisorption bond with a metal spin-orbital will depend on whether the spins are aligned antiparallel (singlet) or parallel (triplet). For achiral molecules, the charge redistribution in the molecule as it approaches and binds to the surface is not spin-dependent, and no apparent spin specificity is observed. As a chiral molecule approaches the surface, however, it undergoes a spin-dependent charge redistribution so that one enantiomer interacts along a singlet reaction coordinate and the other enantiomer interacts along a triplet reaction coordinate. This mechanism for enantiospecific interaction of chiral molecules and ferromagnetic surfaces was not appreciated previously.
If a ferromagnetic substrate is not yet magnetized, the surface comprises a mixture of domains magnetized up or down; on average the density of states for spin pointing “up” or “down” is the same. The electronic interaction at the interface between chiral molecules and the ferromagnetic substrate, the spinterface,23 breaks the spin degeneracy so that one spin is preferred over the other, depending on the handedness of the adsorbed chiral molecules. The exchange interaction of a chiral oligopeptide with a magnetic substrate is strong enough to switch and control the magnetization direction of the substrate.24
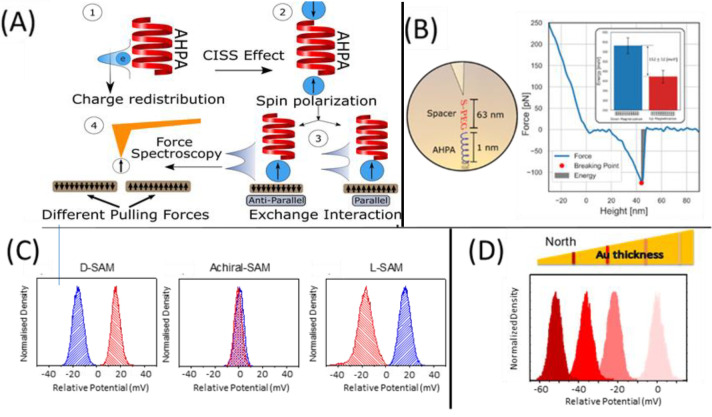
A direct measurement of the exchange energies that control the interaction between a ferromagnetic substrate and chiral molecules was realized by atomic force mapping, as presented in the top panel of Figure 3.4 In this experiment, a helical (and chiral) polypeptide, α-helix polyalanine, was adsorbed on the gold cantilever of an atomic force microscope and then used to measure the force between the molecule and a ferromagnetic substrate (Figure 3B, left). As the cantilever (and molecule) approach the substrate, the spin dependence for the chiral molecule/surface interaction manifests in a different force acting on the AFM cantilever (Figure 3A). Integrating the force over the tip displacement provides an energy for the interaction, and the difference in energy for the two magnetization directions (up versus down) of the ferromagnetic (FM) substrate reports on the difference in the interaction energy of the chiral molecule and the magnetized sample. These experiments find an energy difference of ∼150 meV at short-range, which is about 6kBT and significantly larger than the expected Zeeman splitting (Figure 3B).
Figure 3.
FM/chiral molecule interactions. (top) Direct measurement of the exchange interaction between the FM and the chiral molecules. (A) α-Helix polyalanine (AHPA) is adsorbed on a gold AFM cantilever. The system is immersed in ethanol to reduce capillary forces. The sample under study is an MBE grown Co-based nanostructure with an out-of-plane easy axis. When the tip is close to the sample, reorganization of the electric charges in the molecule (1) results in spin filtering due to the CISS effect (2), which is followed by an exchange interaction between the molecular wave function and the wave function of the substrate (3). This interaction is sensed by the deflection of the AFM cantilever (4). (B) (left) Schematic of the tip with the adsorbed molecules; (right) typical force dependence on the tip–surface distance; the pulling point of the molecule and the integrated area represent the pulling energy of the molecule. Inset shows the mean pulling energy for the up and down direction of perpendicular sample magnetization, showing a difference of 150 meV. The standard error of the mean is shown. Reprinted from ref (4) with permission. Copyright 2019 Wiley-VCH. (bottom) Contact potential difference for chiral and achiral self-assembled monolayers on magnetized surfaces. (C) Histograms, obtained from Kelvin probe measurements of the contact potential difference (CPD) for chiral and achiral self-assembled monolayers on Ni/Au magnetized surfaces, reveal an enantiospecific response for chiral molecules and no magnetization response for achiral molecules. (D) Change in the CPD as a function of the Au layer thickness for Co magnetized films with adsorbed L-A5 (SH-(CH2)2-NH-(Ala-Aib)5-COOH) SAMs. The top diagram represents the Au wedge, and the color of each plot corresponds to the region indicated on the gradient bar by the same shade. Reprinted with permission from ref (18). Copyrights 2020 American Chemical Society.
To quantify the work function differences in the chiral molecule–FM system, Kelvin-probe measurements on ferromagnetic thin film electrodes coated with self-assembled monolayers of chiral molecules were performed.18 These measurements, which are presented in the bottom panel of Figure 3, reveal that the electron penetration from the metal electrode into the chiral molecules depends on the ferromagnet’s magnetization direction and the molecules’ chirality. The Kelvin probe microscopy data show that the extent to which the electron density from the ferromagnet penetrates into the chiral peptide monolayer is enantiospecific and changes with the sign of the magnetization; that is, tunneling into chiral molecules is spin dependent (Figure 3C). Figure 3D shows that the enantiospecific interaction of chiral molecules with a ferromagnetic surface depends on the amplitude of the electron spin wave function penetration. Together, these data and other findings from these studies show that as chiral molecules adsorb on the surface of thin ferromagnetic films, a magnetization perpendicular to the surface is created, without the application of current or an external magnetic field.24 Correspondingly, changes in the surface magnetization generate changes in the electrostatic field for chiral molecules. Although the energy difference has contributions from magnetic field (Zeeman) effects, it is dominated by the spin exchange energy.
Controlling Chemical Reaction by Spin: Water Oxidation
Despite significant progress in recent years, inefficiencies in the oxygen evolution reaction (OER) remain an important barrier to the broad deployment of water electrolysis.25,26 Three primary obstacles arising from the OER are (i) required overpotentials of about 0.6 V, (ii) production of H2O2 byproduct,27−29 and (iii) the need to operate at high pH (>10), which can lead to chemical corrosion of device components. The H2O2 production can be reduced and the energy efficiency improved by better controlling the spin of the electrons injected into the anode.30−33
Control over spin in the elementary electrochemical steps generates spin alignment in the unpaired electrons of the intermediate radicals (•OH) that are adsorbed on the surface and combine along a triplet surface to form the ground-state triplet oxygen molecule (3O2). Because hydrogen peroxide is a singlet species, polarizing the spin of •OH inhibits H2O2 evolution and reduces the overpotential. The benefits of spin control in the electrolysis have been shown by using chiral anode materials and by using magnetized anodes. Studies with a TiO2 anode that was modified with a monolayer of chiral molecules, chiral supramolecular structures, or a chiral film were reported, as well as anodes comprising chiral inorganic oxides of CuO34 and CoOx (both paramagnetic and ferromagnetic).35 Spin control has also been demonstrated for magnetic electrodes with high current density and low byproduct yields.36
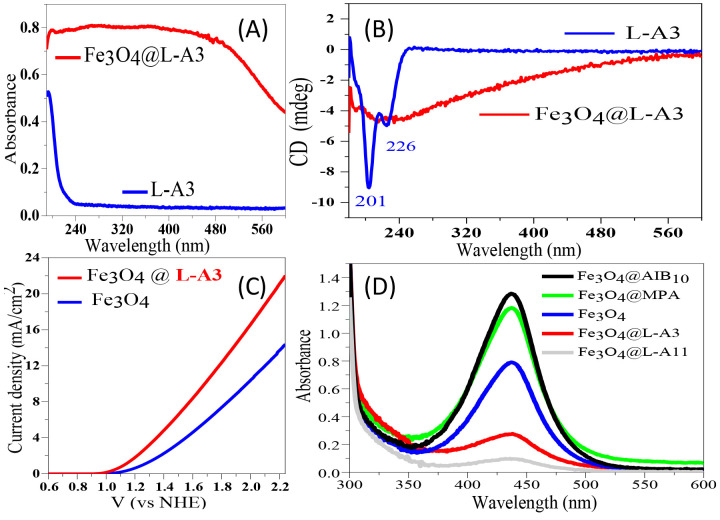
To illustrate the benefit of spin control on the performance of an OER anode, we chose a well-established and efficient OER catalyst, Fe3O4, and converted it into a chiral catalyst by chemisorbing chiral molecules onto it.37Figure 4 shows the absorption (A) and the circular dichroism spectra (B) of the peptide SH-(CH2)2-NH-(Ala-Aib)3-COOH (L-A3) and the Fe3O4@L-A3 NPs in solution. The CD spectrum of L-A3 in (Figure 4B) exhibits two CD peaks at 201 and 226 nm. Attaching L-A3 to Fe3O4 results in a CD spectrum in the range of the Fe3O4 NPs’ absorption. This effect of induced chirality in NPs, as a result of the adsorption of chiral molecules, is well documented38−40 and is demonstrated here for the Fe3O4 NPs.41,42 It was observed for all chiral molecule@Fe3O4 NPs studied in the present work. In the past, it has been shown that electron transfer through semiconducting NPs coated with chiral molecules is spin dependent.43,44
Figure 4.
Spin controlled water splitting. The results obtained using 20 nm Fe3O4 nanoparticles and nanoparticles coated with achiral or chiral molecules, which are supported on an FTO substrate to make the OER anode. (A) Absorption spectra of a 1 mM L-A3 solution (blue line) and Fe3O4@L-A3 (red line); (B) CD spectra of a 1 mM solution of the molecules (blue line) and Fe3O4 particles to which the chiral molecules were attached (red line). (C) Current density of Fe3O4 NPs linked with L-A3 (chiral) and pure Fe3O4 NPs. (D) Visible absorption spectra from the titration of the electrolyte used (0.1 M Na2SO4) with o-tolidine of bare Fe3O4, Fe3O4@L-A11 (chiral), Fe3O4@L-A3 (chiral), Fe3O4@MPA (achiral), and Fe3O4@AIB10 (NH-(CH2)2-SH-(Aib)10-NH2, achiral). The absorption scale in (A) and (D) is arbitrary. Reprinted with permission from ref (37). Copyright 2018 American Chemical Society.
Figure 4C shows that the anode currents of Fe3O4 NPs coated with chiral molecules are much larger than those for bare Fe3O4 NPs. Thus, chiral molecules enhance the anode current for water splitting. As expected, there is little difference in performance if l or d enantiomers are used, because either of these choices lead to spin alignment of the radical intermediates on the surface; the correlation of the spin alignment among the radicals is important, not the specific direction of the spins in the laboratory frame.
To confirm the role of CISS-based spin polarization for the anode reaction, we investigated the formation of H2O2 during water electrolysis with these anodes. In these studies, o-tolidine was used as a redox indicator for the presence of H2O2, as it displays an absorption peak at about 436 nm upon reaction with H2O2. Figure 4D shows spectra taken of solutions after the reaction was carried out in a 0.1 M Na2SO4 electrolyte solution. A strong absorbance appeared at 436 nm when the anode was coated with achiral Fe3O4 NPs, namely, the Fe3O4@MPA NPs and Fe3O4@AIB10NPs, and the absorbance was much weaker when the chiral Fe3O4 NPs (Fe3O4@L-A11 and Fe3O4@L-A3) were used on the anode. Thus, the chiral imprinted Fe3O4 NPs inhibit H2O2 formation. Moreover, Figure 4D shows that Fe3O4@L-A11 exhibited better performance in suppressing the production of H2O2 than does Fe3O4@L-A3. This phenomenon demonstrates that the L-A11 molecules have a stronger CISS response than the L-A3 molecules; their longer chains provide higher spin selectivity.15
The data in Figure 4 demonstrate the importance of spin control for reducing the reaction barrier and reducing byproduct formation in the OER reaction. In this example, the chirality was imprinted on the NPs by a chiral molecule ligand shell, which also facilitates spin-polarized intermediates because of the CISS-based spin filtering. The concepts illustrated by the results in Figure 4, and our earlier work, have been corroborated in other studies45,46 and for anodes operating at much higher current densities. For example, Garces-Pineda and co-workers showed that an external magnetic field increases the current densities by two times at 100 mA cm–2 under high applied bias,47 and other workers have shown that the magnetization state of ferromagnetic catalysts affect the OER efficiency.48,49 In a recent study, significant improvements were demonstrated in current density for chiral CoOx films over their achiral analogues at current densities of 50 mA/cm2. Lastly, a number of theoretical studies have identified the importance of spin considerations during O2(3Σg–) generation.50−53 It must be appreciated that the spin selectivity is a result of the reactants being adsorbed on the electrodes and therefore not being able to rotate freely.
The studies of chiral catalysts on the oxygen evolution reaction show that the spin polarization of intermediates (which arises because of CISS effects) can be used to select the desired reaction pathway(s) over others.
CISS and Enantioselective Electrochemistry
In addition to improved product selectivity because of spin selection rules, CISS concepts can be applied to enantioselective electrochemical reactions. Recently, we showed that magnetized film electrodes can be used to perform enantioselective electrochemistry.54 In one case, we used magnetized electrodes to selectively decompose one enantiomer over the other in a racemic mixture. In a second case, we showed that the spin polarization that is introduced into an achiral monolayer film by a magnetized substrate enantiospecifically binds one enantiomer from a racemic mixture, thus creating a chiral adduct between molecules in a racemic mixture and a magnetized substrate. The methods illustrated by these two approaches could be used in enantioselective synthesis as well as chiral resolution. In a third case, we used a magnetized substrate to electropolymerize chiral polymer films on a magnetized electrode. In this case the monomer reactants in the electropolymerization are achiral and the CISS selectivity is believed to arise during the propagation phase of the polymer growth rather than in the initiation phase. A more detailed review on this work has recently appeared, and the reader is referred to that for more details.9
Future Perspective
The field of spin selective Chemistry with chiral molecules is in its infancy. As illustrated by the water electrolysis studies with chiral electrodes, controlling the electron spin polarization of adsorbed intermediates affects the reactivity and changes the reaction product outcomes. As an extension of the work performed on the oxidation of water, other multielectron reactions should be studied, in which the product is in its triplet state, like oxygen, or when the byproduct is oxygen. Controlling the spin in these reactions may affect the reaction barriers and change the ratio between different products. Particularly interesting and important examples for such reactions may prove to be the reduction of CO2 or N2 because of their importance to solar fuels chemistry.
The work described above makes clear that the conventional wisdom as to the electronic nature of a “closed shell” molecular system is misleading when applied to chiral molecules. Spin control has the potential to transform the synthesis of chiral molecules. Although not yet tested, the enantioselective reduction of prochiral molecules to chiral products on a ferromagnetic surface that is coated with chiral molecules that serve as an asymmetric catalyst could prove to be a general strategy. It is interesting to note that the chiral molecules attached to the surface need not be enantiopure, because the selectivity will be achieved by the direction of magnetization of the substrate, which causes the emission of electrons with their spins polarized either parallel or antiparallel to their momentum (see Figure 1).
The findings described here should also change the way one calculates interactions between chiral molecules. As a result of the SDCR effect, the interaction between two chiral molecules or between chiral molecules and a ferromagnetic surface should include the spin exchange interaction. Because of the large magnitude of this term, even relatively small spin polarizations of a few percent may result in significant interaction energies. This can be important for Biology as most interaction and conformational adjustments are associated with chiral structures.
Acknowledgments
The authors acknowledge the partial support of the John Templeton Foundation. R.N. and D.H.W. acknowledge the partial support of the US Department of Energy Grant No. ER46430 and the NSF-BSF-1852588. R.N. and Y.P. acknowledge the partial support from the Israel Ministry of Science.
Biographies
Ron Naaman is Professor Emeritus from the Weizmann Institute. He received his Ph.D. from the same institute and spent two years at Stanford as a postdoctoral fellow and an additional year at Harvard University. His research involves the study of the interaction of electrons with organic molecules and biosystems like proteins, oligopeptides, and DNA. His research group discovered the chiral induced spin selectivity effect in collaboration with David Waldeck. This effect has been the focus of his research for the last two decades.
Yossi Paltiel is a Professor in the Applied Physics Department in the Hebrew University of Jerusalem Israel. Prof. Paltiel has worked both for leading high-tech industry groups and in the academic world. The Paltiel group’s goal is to establish a way to incorporate quantum mechanics into room temperature devices mimicking Biology and Chemistry processes. In this sense, the group also works on spin interfaces using chiral molecules and materials. Paltiel has two startup companies.
David H. Waldeck is a Professor of Chemistry and the Academic Director of the Petersen Institute of NanoScience and Engineering at the University of Pittsburgh. He received his Ph.D. in Chemistry from the University of Chicago and then held an IBM postdoctoral fellowship at U.C., Berkeley, before moving to Pittsburgh. His research uses methods of spectroscopy, electrochemistry, and microscopy to investigate primary processes in molecules, supramolecular assemblies, and nanomaterials. Currently, his research is focused on the fundamental understanding of the chiral induced spin selectivity (CISS) effect, as well as its role in electron transfer reactions and electron transport in supramolecular structures.
The authors declare no competing financial interest.
References
- Rosenberg R.; Abu Haija M.; Ryan P. J. Chiral-selective Chemistry Induced by Spin-polarized Secondary Electrons from a Magnetic Substrate. Phys. Rev. Lett. 2008, 101, 178301. 10.1103/PhysRevLett.101.178301. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Capua E.; Kesharwani M. K.; Martin J. M. L.; Sitbon E.; Waldeck D. H.; Naaman R. Chirality-induced Spin Polarization Places Symmetry Constraints on Biomolecular Interactions. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 2474–2478. 10.1073/pnas.1611467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee-Ghosh K.; Ben Dor O.; Tassinari F.; Capua E.; Yochelis S.; Capua A.; Yang S.-H.; Parkin S. S. P.; Sarkar S.; Kronik L.; Baczewski L. T.; Naaman R.; Paltiel Y. Separation of Enantiomers by Their Enantiospecific Interaction with Achiral Magnetic Substrates. Science 2018, 360, 1331–1334. 10.1126/science.aar4265. [DOI] [PubMed] [Google Scholar]
- Ziv A.; Saha A.; Alpern H.; Sukenik N.; Baczewski L. T.; Yochelis S.; Reches M.; Paltiel Y. AFM-Based Spin Exchange Microscopy Using Chiral Molecules. Adv. Mater. 2019, 31, 1904206. 10.1002/adma.201904206. [DOI] [PubMed] [Google Scholar]
- Steiner U. E.; Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. 10.1021/cr00091a003. [DOI] [Google Scholar]
- Hore P. J.; Ivanov K. L.; Wasielewski M. R. Spin chemistry. J. Chem. Phys. 2020, 152, 120401. 10.1063/5.0006547. [DOI] [PubMed] [Google Scholar]
- Hayashi H.Introduction to Dynamic Spin Chemistry; World Scientific Publisher, Singapore, 2004. [Google Scholar]
- Kalogerakis K. S.; Zare R. N. Energy and angular momentum control of the specific opacity functions in the Ba+HI→BaI+H reaction. J. Chem. Phys. 1996, 104, 7947. 10.1063/1.471511. [DOI] [Google Scholar]
- Bloom B. P.; Lu Y.; Waldeck D. H.; Metzger T.; Yochelis S.; Paltiel Y.; Fontanesi C.; Mishra S.; Tassinari F.; Naaman R. Asymmetric Reactions Induced by Electron Spin Polarization. Phys. Chem. Chem. Phys. 2020, 22, 21570–21582. 10.1039/D0CP03129A. [DOI] [PubMed] [Google Scholar]
- Evans E. W.; Dodson C. A.; Maeda K.; Biskup T.; Wedge C. J.; Timmel C. R. Magnetic field effects in flavoproteins and related systems. Interface Focus 2013, 3, 20130037. 10.1098/rsfs.2013.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe G.; Norris J. R.; Poluektov O. G.; Thurnauer M. C. Chapter 15: High-Time Resolution Electron Paramagnetic Resonance Study of Quantum Beat Oscillations Observed in Photosynthetic Reaction Center Proteins. Adv. Photosynth. Respir. 2008, 26, 305–323. 10.1007/978-1-4020-8250-4_15. [DOI] [Google Scholar]
- Hiscock H. G.; Worster S.; Kattnig D. R.; Steers C.; Jin Y.; Manolopoulos D. E.; Mouritsen H.; Hore P. J. The quantum needle of the avian magnetic compass. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 4634–4639. 10.1073/pnas.1600341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turro N. J. The role of intersystem crossing steps in singlet oxygen chemistry and photo-oxidations. Tetrahedron 1985, 41, 2089–2098. 10.1016/S0040-4020(01)96579-2. [DOI] [Google Scholar]
- Buchachenko A. L. Recent advances in spin chemistry. Pure Appl. Chem. 2000, 72, 2243–2258. 10.1351/pac200072122243. [DOI] [Google Scholar]
- Michaeli K.; Kantor-Uriel N.; Naaman R.; Waldeck D. H. The Electron’s Spin and Molecular Chirality- How Are They Related and How Do They Affect Life Processes?. Chem. Soc. Rev. 2016, 45, 6478–6487. 10.1039/C6CS00369A. [DOI] [PubMed] [Google Scholar]
- Ray K.; Ananthavel S. P.; Waldeck D. H.; Naaman R. Asymmetric scattering of polarized electrons by organized organic films made of chiral molecules. Science 1999, 283, 814. 10.1126/science.283.5403.814. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Paltiel Y.; Waldeck D. H. A Perspective on Chiral Molecules and the Spin Selectivity Effect. J. Phys. Chem. Lett. 2020, 11, 3660–3666. 10.1021/acs.jpclett.0c00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Mishra S.; Avigad E.; Bloom B. P.; Baczewski L. T.; Yochelis S.; Paltiel Y.; Naaman R.; Waldeck D. H. Effect of Chiral Molecules on the Electron’s Spin Wavefunction at Interfaces. J. Phys. Chem. Lett. 2020, 11, 1550. 10.1021/acs.jpclett.9b03487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden E.Hall-Effect Sensors: Theory and Application; Elsevier, 2011. [Google Scholar]
- Kumar A.; Capua E.; Vankayala K.; Fontanesi C.; Naaman R. Magnetless Device for Conducting Three-Dimensional Spin-Specific Electrochemistry. Angew. Chem., Int. Ed. 2017, 56, 14587. 10.1002/anie.201708829. [DOI] [PubMed] [Google Scholar]
- Mishra S.; Mondal A. K.; Smolinsky E. Z. B.; Naaman R.; Maeda K.; Nishimura T.; Taniguchi T.; Yoshida T.; Takayama K.; Yashima E. Spin Filtering Along Chiral Polymers. Angew. Chem., Int. Ed. 2020, 59, 14671. 10.1002/anie.202006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolinsky E. Z. B.; Neubauer A.; Kumar A.; Yochelis S.; Capua E.; Carmieli R.; Paltiel Y.; Naaman R.; Michaeli K. Electric Field Controlled Magnetization in GaAs/AlGaAs Heterostructures-chiral Organic Molecules Hybrids. J. Phys. Chem. Lett. 2019, 10, 1139–1145. 10.1021/acs.jpclett.9b00092. [DOI] [PubMed] [Google Scholar]
- Cinchetti M.; Dediu V. A.; Hueso L. E. Activating the Molecular Spinterface. Nat. Mater. 2017, 16, 507–515. 10.1038/nmat4902. [DOI] [PubMed] [Google Scholar]
- Ben Dor O.; Yochelis S.; Radko A.; Vankayala K.; Capua E.; Capua A.; Yang S.-H.; Baczewski L. T.; Parkin S. S. P.; Naaman R.; Paltiel Y. Magnetization switching in ferromagnets by adsorbed chiral molecules without current or external magnetic field. Nat. Commun. 2017, 8, 14567. 10.1038/ncomms14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graetzel M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Shin H. S.; Loh K. P.; Chhowalla M. Low-Dimensional Catalysts for Hydrogen Evolution and CO2 Reduction. Nat. Rev. Chem. 2018, 2, 0105. 10.1038/s41570-017-0105. [DOI] [Google Scholar]
- Liu J.; Liu Y.; Liu N.; Han Y.; Zhang X.; Huang H.; Lifshitz Y.; Lee S.; Zhong J.; Kang Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science 2015, 347 (6225), 970–974. 10.1126/science.aaa3145. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhang Y.; Lu L.; Chen W.; Wu G. Self-Regenerated Solar-Driven Photocatalytic Water-Splitting by Urea Derived Graphitic Carbon Nitride with Platinum Nanoparticles. Chem. Commun. 2012, 48, 8826–8828. 10.1039/c2cc33644h. [DOI] [PubMed] [Google Scholar]
- Seabold J. A.; Choi K.-S. Effect of a Cobalt-Based Oxygen Evolution Catalyst on the Stability and the Selectivity of Photo-Oxidation Reactions of a WO3 Photoanode. Chem. Mater. 2011, 23, 1105–1112. 10.1021/cm1019469. [DOI] [Google Scholar]
- Mtangi W.; Kiran V.; Fontanesi C.; Naaman R. Role of the Electron Spin Polarization in Water Splitting. J. Phys. Chem. Lett. 2015, 6, 4916–4922. 10.1021/acs.jpclett.5b02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtangi W.; Tassinari F.; Vankayala K.; Vargas Jentzsch A.; Adelizzi B.; Palmans A. R. A.; Fontanesi C.; Meijer E. W.; Naaman R. Control of Electrons’ Spin Eliminates Hydrogen Peroxide Formation During Water Splitting. J. Am. Chem. Soc. 2017, 139, 2794–2798. 10.1021/jacs.6b12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal P. C.; Mtangi W.; Fontanesi C. Chiro-Spintronics: Spin-Dependent Electrochemistry and Water Splitting Using Chiral Molecular Films. Small Methods 2018, 2, 1700313. 10.1002/smtd.201700313. [DOI] [Google Scholar]
- Tassinari F.; Banerjee-Ghosh K.; Parenti F.; Kiran V.; Mucci A.; Naaman R. Enhanced Hydrogen Production with Chiral Conductive Polymer-Based Electrodes. J. Phys. Chem. C 2017, 121, 15777–15783. 10.1021/acs.jpcc.7b04194. [DOI] [PubMed] [Google Scholar]
- Ghosh K. B.; Zhang W.; Tassinari F.; Mastai Y.; Lidor-Shalev O.; Naaman R.; Möllers P.; Nürenberg D.; Zacharias H.; Wei J.; Wierzbinski E.; Waldeck D. H. Controlling Chemical Selectivity in Electrocatalysis with Chiral CuO Coated Electrodes. J. Phys. Chem. C 2019, 123, 3024–3031. 10.1021/acs.jpcc.8b12027. [DOI] [Google Scholar]
- Ghosh S.; Bloom B. P.; Lu Y.; Lamont D.; Waldeck D. H. Increasing the Efficiency of Water Splitting through Spin Polarization using Cobalt Oxide Thin Film Catalysts. J. Phys. Chem. C 2020, 10.1021/acs.jpcc.0c07372. [DOI] [Google Scholar]
- Garcés-Pineda F. A.; Blasco-Ahicart M.; Nieto-Castro D.; López N.; Galán-Mascarós J. R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy. 2019, 4, 519–525. 10.1038/s41560-019-0404-4. [DOI] [Google Scholar]
- Zhang W.; Banerjee-Ghosh K.; Tassinari F.; Naaman R. Enhanced Electrochemical Water Splitting with Chiral Molecule-coated Fe3O4 Nano-particles. ACS Energy Lett. 2018, 3, 2308–2313. 10.1021/acsenergylett.8b01454. [DOI] [Google Scholar]
- Moloney M. P.; Gun’ko Y. K.; Kelly J. M. Chiral Highly Luminescent CdS Quantum Dots. Chem. Commun. 2007, 0 (38), 3900–3902. 10.1039/b704636g. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R.; George C. B.; Zuber G.; Naaman R.; Waldeck D. H.; Wipf P.; Beratan D. N. The Chiroptical Signature of Achiral Metal Clusters Induced by Dissymmetric Adsorbates. Phys. Chem. Chem. Phys. 2006, 8, 63–67. 10.1039/B511563A. [DOI] [PubMed] [Google Scholar]
- Ma W.; Xu L.; de Moura A. F.; Wu X.; Kuang H.; Xu C.; Kotov N. A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. 10.1021/acs.chemrev.6b00755. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhou J.; Deng J. Helical polymer/Fe3O4 NPs Constructing Optically Active, Magnetic Core/shell Microspheres: Preparation by Emulsion Polymerization and Recycling Application in Enantioselective Crystallization. Polym. Chem. 2016, 7, 125–134. 10.1039/C5PY01549A. [DOI] [Google Scholar]
- Safaei-Ghomi J.; Zahedi S. L-Proline-functionalized Fe3O4 Nanoparticles as a Novel Magnetic Chiral Catalyst for the Direct Asymmetric Mannich Reaction. Appl. Organomet. Chem. 2015, 29, 566–571. 10.1002/aoc.3333. [DOI] [Google Scholar]
- Ben Dor O.; Morali N.; Yochelis S.; Paltiel Y.; Baczewski L. T. Local Light-Induced Magnetization Using Nanodots and Chiral Molecules. Nano Lett. 2014, 14, 6042–6049. 10.1021/nl502391t. [DOI] [PubMed] [Google Scholar]
- Bloom B. P.; Kiran V.; Varade V.; Naaman R.; Waldeck D. H. Spin Selective Charge Transport through Cysteine Capped CdSe Quantum Dots. Nano Lett. 2016, 16, 4583–4589. 10.1021/acs.nanolett.6b01880. [DOI] [PubMed] [Google Scholar]
- Pan L.; Ai M.; Huang C.; Yin L.; Liu X.; Zhang R.; Wang S.; Jiang Z.; Zhang X.; Zou J.-J.; Mi W. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 2020, 11, 418. 10.1038/s41467-020-14333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y.; Sharpe R.; Lim T.; Niemantsverdriet J. W. H.; Gracia J. Photosystem II Acts as a Spin-Controlled Electron Gate during Oxygen Formation and Evolution. J. Am. Chem. Soc. 2017, 139, 16604–16608. 10.1021/jacs.7b07634. [DOI] [PubMed] [Google Scholar]
- Garces-Pineda F. A.; Blasco-Ahicart M.; Nieto-Castro D.; Lopez N.; Galan-Mascaros J. R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy. 2019, 4, 519–525. 10.1038/s41560-019-0404-4. [DOI] [Google Scholar]
- Yang Z.; Liang X. Self-magnetic-attracted NixFe(1-x)@NixFe(1-x)O nanoparticles on nickel foam as highly active and stable electrocatalysts towards alkaline oxygen evolution reaction. Nano Res. 2020, 13, 461–466. 10.1007/s12274-020-2630-2. [DOI] [Google Scholar]
- Davodi F.; Muhlhausen E.; Tavakkoli M.; Sainio J.; Jiang H.; Gokce B.; Marzun G.; Kallio T. Catalyst Support Effect on the Activity and Durability of Magnetic Nanoparticles: toward Design of Advanced Electrocatalyst for Full Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 31300–31311. 10.1021/acsami.8b08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien S.; Metiu H. O2 evolution on a clean partially reduced rutile TiO2(110) surface and on the same surface precovered with Au1 and Au2: The importance of spin conservation. J. Chem. Phys. 2008, 129, 074705. 10.1063/1.2956506. [DOI] [PubMed] [Google Scholar]
- Torun E.; Fang C. M.; de Wijs G. A.; de Groot R. A. Role of Magnetism in Catalysis: RuO2 (110) Surface. J. Phys. Chem. C 2013, 117, 6353–6357. 10.1021/jp4020367. [DOI] [Google Scholar]
- Sharpe R.; Munarriz J.; Lim T.; Jiao Y.; Niemantsverdriet J. W.; Polo V.; Gracia J. Orbital physics of perovskites for the oxygen evolution reaction. Top. Catal. 2018, 61, 267–275. 10.1007/s11244-018-0895-4. [DOI] [Google Scholar]
- Gracia J. Spin dependent interactions catalyse the oxygen electrochemistry. Phys. Chem. Chem. Phys. 2017, 19, 20451. 10.1039/C7CP04289B. [DOI] [PubMed] [Google Scholar]
- Metzger T. S.; Mishra S.; Bloom B. P.; Goren N.; Neubauer A.; Shmul G.; Wei J.; Yochelis S.; Tassinari F.; Fontanesi C.; Waldeck D. H.; Paltiel Y.; Naaman R. The Electron Spin as a Chiral Reagent. Angew. Chem., Int. Ed. 2020, 59, 1653–1658. 10.1002/anie.201911400. [DOI] [PubMed] [Google Scholar]